(S)-tert-LeucinolCAS# 112245-13-3 |
Quality Control & MSDS
Number of papers citing our products

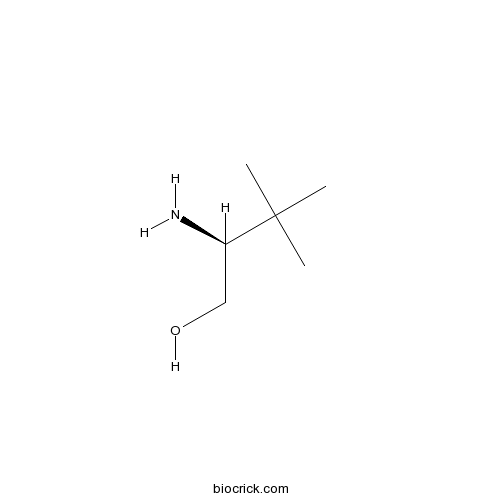
Chemical structure

3D structure
| Cas No. | 112245-13-3 | SDF | Download SDF |
| PubChem ID | 2734079 | Appearance | Powder |
| Formula | C6H15NO | M.Wt | 117.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-amino-3,3-dimethylbutan-1-ol | ||
| SMILES | CC(C)(C)C(CO)N | ||
| Standard InChIKey | JBULSURVMXPBNA-RXMQYKEDSA-N | ||
| Standard InChI | InChI=1S/C6H15NO/c1-6(2,3)5(7)4-8/h5,8H,4,7H2,1-3H3/t5-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(S)-tert-Leucinol Dilution Calculator

(S)-tert-Leucinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.5332 mL | 42.6658 mL | 85.3315 mL | 170.663 mL | 213.3288 mL |
| 5 mM | 1.7066 mL | 8.5332 mL | 17.0663 mL | 34.1326 mL | 42.6658 mL |
| 10 mM | 0.8533 mL | 4.2666 mL | 8.5332 mL | 17.0663 mL | 21.3329 mL |
| 50 mM | 0.1707 mL | 0.8533 mL | 1.7066 mL | 3.4133 mL | 4.2666 mL |
| 100 mM | 0.0853 mL | 0.4267 mL | 0.8533 mL | 1.7066 mL | 2.1333 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- (R)-(-)-Modafinic acid
Catalog No.:BCC5157
CAS No.:112111-45-2
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
- Endoxifen
Catalog No.:BCC7761
CAS No.:112093-28-4
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- XL765
Catalog No.:BCC2060
CAS No.:1123889-87-1
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
- 3-Deoxysappanchalcone
Catalog No.:BCN3736
CAS No.:112408-67-0
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
Diastereo- and enantioselective cyclopropanation with chromium fischer carbene complexes: alkenyl oxazolines as useful achiral and chiral substrates.[Pubmed:11673980]
J Am Chem Soc. 2001 Oct 31;123(43):10494-501.
The cyclopropanation reaction of chromium Fischer carbene complexes with alkenyl oxazolines has been studied in both racemic and enantioselective fashions. The oxazolinyl group acts as both electron-acceptor substituent and chiral auxiliary. Achiral (4,4-dimethyloxazolin-2-yl)alkenes derived from trans-crotonic and trans-cinnamic acids 2a,b undergo the cyclopropanation reaction to give 4a-d,g with excellent diastereoselectivity (trans/cis ratio between 93:7 and >97:3), while those derived from acrylic and metacrylic acids 2c,d give the cyclopropanes 4e,f,h with much lower selectivity (trans/cis ratio between 68:32 and 83:17). The homogeneous catalytic hydrogenolysis of 4 leads in a selective manner to 5 or 6, depending on the nature of the R3 substituent. The removal of the oxazoline moiety is achieved by carboxybenzylation/hydrolysis and ester reduction, yielding monoprotected 1,4- and 1,3-diols 9 and 11, respectively. The alkenes derived from enantiopure (S)-valinol and (S)-tert-Leucinol 3 led to cyclopropanes trans-12 with high relative and absolute stereocontrol. Using tert-leucinol as the auxiliary permits attaining total facial stereoselectivity (>98% ee). Reductive cleavage of the cyclopropane ring and removal of the auxiliary afford the enriched alcohols (3S,4S)-9 and (S)-11. The stereochemical outcome of the cyclopropanation reaction is rationalized by a trans approach of the s-cis conformer of the alkenyl oxazoline to the carbene complex involving the less hindered face of the oxazoline auxiliary and the re-face of the carbene complex.
Chiral carbene-borane adducts: precursors for borenium catalysts for asymmetric FLP hydrogenations.[Pubmed:27383522]
Dalton Trans. 2016 Oct 21;45(39):15303-15316.
The carbene derived from (1R,3S)-camphoric acid was used to prepare the borane adduct with Piers' borane 7. Subsequent hydride abstraction gave the borenium cation 8. Adducts with 9-BBN and the corresponding (1R,3S)-camphoric acid-derived carbene bearing increasingly sterically demanding N-substituents (R = Me 9, Et 10, i-Pr 11) and the corresponding borenium cations 12-14 were also prepared. These cations were not active as catalysts in hydrogenation, although 9-11 were shown to undergo carbene ring expansion reactions at 50 degrees C to give species 15-17. The IBOX-carbene precursors 18 and 19 derived from amino alcohols (S)-valinol and (S)-tert-Leucinol (R = i-Pr, t-Bu) were used to prepare borane adducts 20-23. Reaction of the carbenes 1,3-dimethylimidazol-2-ylidene (IMe), 1,3-di-iso-propylimidazol-2-ylidene (IPr) 1-benzyl-3-methylimidazol-2-ylidene (IBnMe), 1-methyl-3-phenylimidazol-2-ylidene (IPhMe) and 1-tert-butyl-3-methylimidazol-2-ylidene (ItBuMe) with diisopinocampheylborane (Ipc2BH) gave chiral adducts: (IMe)(Ipc2BH) 24, (IPr)(Ipc2BH) 25, (IBnMe)(Ipc2BH) 26, (IPhMe)(Ipc2BH) 27, and (ItBuMe)(Ipc2BH) 28. Triazolylidene-type adducts including the (10)-phenyl-9-borabicyclo [3.3.2]decane adduct of 1,3,4-triphenyl-1H-1,2,3-triazolium, rac-29 and the 9-BBN derivative of (S)-2-amino-2'-methoxy-1,1'-binaphthalene-1,2,3-triazolium 34a/b were also prepared. In catalytic studies of these systems, while several species were competent catalysts for imine reduction, in general, low enantioselectivities, ranging from 1-20% ee, were obtained. The implications for chiral borenium cation catalyst design are considered.
Enantioseparation of racemic N-acylarylalkylamines on various amino alcohol derived tau-acidic chiral stationary phases.[Pubmed:12613838]
J Chromatogr A. 2003 Feb 14;987(1-2):429-38.
Five tau-acidic chiral stationary phases (CSPs), CSP 4, CSP 5, CSP 6, CSP 7 and CSP 8, were prepared by connecting the N-(3,5-dimethylbenzoyl) derivative of (R)-alaninol, (S)-leucinol, (1S,2R)-ephedrine and (S)-tert-Leucinol and the O-(3,5-dinitrobenzoyl) derivative of (R)-phenylglycinol to silica gel through a carbamate or urea linkage. The CSPs were applied to the resolution of various racemic N-acyl-1-naphthylaminoalkanes by chiral HPLC, and the chromatographic resolution results were compared with those of previously reported CSPs (CSP 2, CSP 3), which are derived from N-(3,5-dinitrobenzoyl)-(1S,2R)-norephedrine and N-(3,5-dinitrobenzoyl-(R)-phenylglycinol. Based on a comparison of the resolution results for each CSP, the role of each functional group on the five chiral selectors is explained.
Vanadium-catalyzed asymmetric oxidation of alpha-hydroxy esters using molecular oxygen as stoichiometric oxidant.[Pubmed:15669834]
J Am Chem Soc. 2005 Feb 2;127(4):1090-1.
A vanadium-catalyzed method for the oxidative kinetic resolution of alpha-hydroxyesters, using oxygen as the terminal oxidant, is described. The catalyst is generated in situ from vanadium(V) tri-iso-propoxyoxide in combination with a tridentate ligand derived from 3,5-di-tert-butylsalicylaldehyde and (S)-tert-Leucinol. The reaction allows for the enantioselective synthesis of both aromatic and aliphatic secondary alcohols, including those containing olefins and alkynes.


