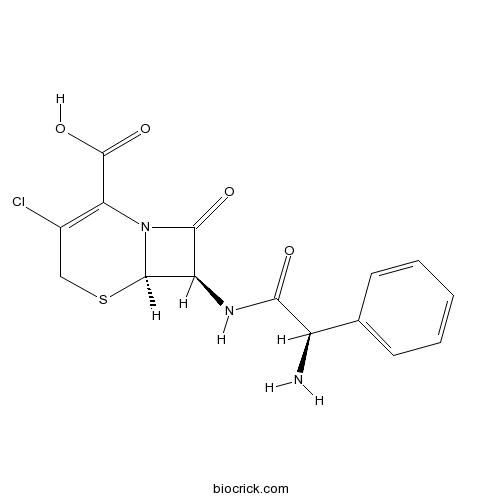
CefaclorCephalosporin antibiotic inhibiting cell wall synthesis. CAS# 53994-73-3 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53994-73-3 | SDF | Download SDF |
| PubChem ID | 51039 | Appearance | Powder |
| Formula | C15H14ClN3O4S | M.Wt | 367.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 18.5 mg/mL (50.30 mM; Need ultrasonic and warming) H2O : 3.85 mg/mL (10.47 mM; Need ultrasonic) | ||
| Chemical Name | (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ||
| SMILES | C1C(=C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)N)C(=O)O)Cl | ||
| Standard InChIKey | QYIYFLOTGYLRGG-GPCCPHFNSA-N | ||
| Standard InChI | InChI=1S/C15H14ClN3O4S/c16-8-6-24-14-10(13(21)19(14)11(8)15(22)23)18-12(20)9(17)7-4-2-1-3-5-7/h1-5,9-10,14H,6,17H2,(H,18,20)(H,22,23)/t9-,10-,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cefaclor, is a second-generation cephalosporin antibiotic used to treat certain infections caused by bacteria such as pneumonia and infections of the ear, lung, skin, throat, and urinary tract.
Target: Antibacterial
Cefaclor belongs to the family of antibiotics known as the cephalosporins (cefalosporins). The cephalosporins are broad-spectrum antibiotics that are used for the treatment of septicaemia, pneumonia, meningitis, biliary tract infections, peritonitis, and urinary tract infections. The pharmacology of the cephalosporins is similar to that of the penicillins, excretion being principally renal. Cephalosporins penetrate the cerebrospinal fluid poorly unless the meninges are inflamed; cefotaxime is a more suitable cephalosporin than cefaclor for infections of the central nervous system, e.g. meningitis. Cefaclor is active against many bacteria, including both Gram-negative and Gram-positive organisms.
Cefaclor is frequently used against bacteria responsible for causing skin infections, otitis media, urinary tract infections, and others. The following represents MIC susceptibility data for a few medically significant microorganisms. Cefaclor is passed into the breast milk in small quantities, but is generally accepted to be safe to take during breastfeeding. Cefaclor is not known to be harmful in pregnancy. Cefaclor has also been reported to cause a serum sickness-like reaction in children. References: | |||||

Cefaclor Dilution Calculator

Cefaclor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7188 mL | 13.594 mL | 27.188 mL | 54.3759 mL | 67.9699 mL |
| 5 mM | 0.5438 mL | 2.7188 mL | 5.4376 mL | 10.8752 mL | 13.594 mL |
| 10 mM | 0.2719 mL | 1.3594 mL | 2.7188 mL | 5.4376 mL | 6.797 mL |
| 50 mM | 0.0544 mL | 0.2719 mL | 0.5438 mL | 1.0875 mL | 1.3594 mL |
| 100 mM | 0.0272 mL | 0.1359 mL | 0.2719 mL | 0.5438 mL | 0.6797 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cefaclor is a cephalosporin antibiotic that inhibits cell wall synthesis. The antibiotic is active against a wide spectrum of common pathogens, including gram-positive and gram-negative bacteria.
- Z-Phg-OH
Catalog No.:BCC2795
CAS No.:53990-33-3
- Luteolin 7,3'-di-O-glucuronide
Catalog No.:BCN5396
CAS No.:53965-08-5
- Ginsenoside F1
Catalog No.:BCN1244
CAS No.:53963-43-2
- Glycyrrhizic acid ammonium salt
Catalog No.:BCN5943
CAS No.:53956-04-0
- Aristolactam BIII
Catalog No.:BCN5718
CAS No.:53948-10-0
- Aristolactam BII
Catalog No.:BCN5717
CAS No.:53948-09-7
- Aristolactam AII
Catalog No.:BCN3924
CAS No.:53948-07-5
- Corylin
Catalog No.:BCN5716
CAS No.:53947-92-5
- Apterin
Catalog No.:BCN3910
CAS No.:53947-89-0
- Euparone
Catalog No.:BCN7204
CAS No.:53947-86-7
- Crotalarine
Catalog No.:BCN2076
CAS No.:53937-97-6
- Deoxyarbutin
Catalog No.:BCC4774
CAS No.:53936-56-4
- L-Nicotine
Catalog No.:BCN6269
CAS No.:54-11-5
- Tryptophan
Catalog No.:BCN2615
CAS No.:54-12-6
- 5-Hydroxyindole-3-Acetic Acid
Catalog No.:BCC8285
CAS No.:54-16-0
- Sodium salicylate
Catalog No.:BCC4846
CAS No.:54-21-7
- Furosemide
Catalog No.:BCC3782
CAS No.:54-31-9
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Idoxuridine
Catalog No.:BCC4666
CAS No.:54-42-2
- Pilocarpine HCl
Catalog No.:BCC4702
CAS No.:54-71-7
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
Immunologic evaluation of immediate hypersensitivity to cefaclor.[Pubmed:25323882]
Yonsei Med J. 2014 Nov;55(6):1473-83.
PURPOSE: Cefaclor is widely prescribed for various infectious diseases. As its consumption increases, the number of hypersensitivity reactions to Cefaclor has increased. This study aimed to evaluate the immunologic findings of immediate hypersensitivity to Cefaclor. MATERIALS AND METHODS: We enrolled 47 patients with immediate hypersensitivity to Cefaclor from Ajou University Hospital and Asan Medical Center. Serum specific IgE, IgG1, and IgG4 antibodies to Cefaclor-human serum albumin (HSA) conjugate were measured by enzyme-linked immunosorbent assay (ELISA). RESULTS: The most common phenotype was anaphylaxis (Group I, 78.7%), followed by urticaria (Group II, 21.3%). The detection of specific IgE, IgG1, and IgG4 to Cefaclor-HSA conjugate by ELISA tended to be higher in Group I (40.5%, 41.7%, 21.6%) than in Group II (20.0%, 20.0%, 0%) with no statistical significance. Significant associations were found between specific IgE and IgG1 or IgG4 (p<0.001, p=0.019). ELISA inhibition tests showed significant inhibitions by both free Cefaclor and Cefaclor-HSA conjugate. For basophil activation tests in patients having no specific IgE antibody, the CD63 expression level on basophils increased with incubations of free Cefaclor. CONCLUSION: The most common manifestation of immediate hypersensitivity to Cefaclor was anaphylaxis, most of which was mediated by IgE; however, a non-IgE mediated direct basophil activation mechanism was suggested in a subset of anaphylaxis patients.
Do We Bury Antibacterials When Launching? Cefaclor Example.[Pubmed:26886327]
J Pharm Sci. 2016 Mar;105(3):1295-300.
This study aimed to compare existing dosing regimens of Cefaclor with recommended pharmacokinetic/pharmacodynamic (PK/PD) parameters and to see if the proposed dosing regimen could have been the reason for development of bacterial resistance. PKs of Cefaclor were determined after administrating the highest therapeutic dose of 750 mg in standard release (SF) and modified release form (MRF) in 12 volunteers. The study was performed on clinical isolates of the most frequent causative agents in urinary and respiratory infections. Minimum inhibitory concentration (MIC), postantibiotic effect, and PK/PD efficacy indices were determined. Peak plasma concentrations of 23.142 +/- 5.67 (SF) and 8.7 +/- 2.09 mug/mL (MRF) were observed after 40-60 min and 3.04 +/- 0.75 h, respectively. MIC for investigated bacterial strains ranged from 1 to 4 mg/L. Postantibiotic effect lasted from 2.10-2.18 +/- 0.2 h for Gram-positive to 0.58-0.90 +/- 0.05 h for Gram-negative bacteria. PK/PD indices (t > MIC) ranged from 27.08 +/- 5.93% to 43.23 +/- 6.54% of 8-h dosing interval (SF) and 22.57 +/- 8.93% to 49.65 +/- 1.95% of 12-h dosing interval (MRF). Plasma levels were below MIC for more than 50% of the dosing interval even for the most sensitive pathogens (MIC = 1 mg/L). During both dosing intervals the total "antibacterial activity" was not longer than 6 h for Gram-positive and 5 h for Gram-negative bacteria for SF and 9 h for Gram-positive and 5 h for Gram-negative bacteria for MRF.


