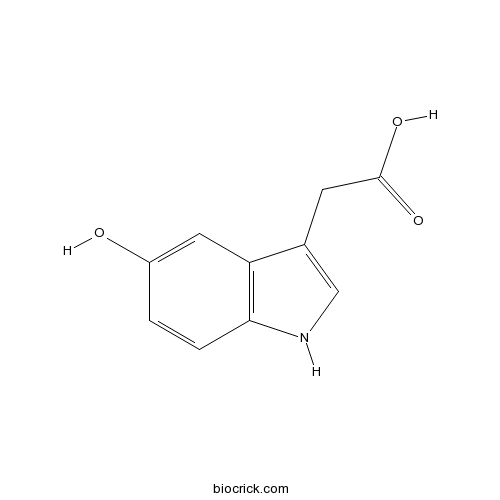
5-Hydroxyindole-3-Acetic AcidCAS# 54-16-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54-16-0 | SDF | Download SDF |
| PubChem ID | 1826 | Appearance | Powder |
| Formula | C10H9NO3 | M.Wt | 191 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Ethanol : 50 mg/mL (261.53 mM; Need ultrasonic) | ||
| Chemical Name | 2-(5-hydroxy-1H-indol-3-yl)acetic acid | ||
| SMILES | C1=CC2=C(C=C1O)C(=CN2)CC(=O)O | ||
| Standard InChIKey | DUUGKQCEGZLZNO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H9NO3/c12-7-1-2-9-8(4-7)6(5-11-9)3-10(13)14/h1-2,4-5,11-12H,3H2,(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Hydroxyindole-3-Acetic Acid Dilution Calculator

5-Hydroxyindole-3-Acetic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2356 mL | 26.178 mL | 52.356 mL | 104.712 mL | 130.8901 mL |
| 5 mM | 1.0471 mL | 5.2356 mL | 10.4712 mL | 20.9424 mL | 26.178 mL |
| 10 mM | 0.5236 mL | 2.6178 mL | 5.2356 mL | 10.4712 mL | 13.089 mL |
| 50 mM | 0.1047 mL | 0.5236 mL | 1.0471 mL | 2.0942 mL | 2.6178 mL |
| 100 mM | 0.0524 mL | 0.2618 mL | 0.5236 mL | 1.0471 mL | 1.3089 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tryptophan
Catalog No.:BCN2615
CAS No.:54-12-6
- L-Nicotine
Catalog No.:BCN6269
CAS No.:54-11-5
- Cefaclor
Catalog No.:BCC2527
CAS No.:53994-73-3
- Z-Phg-OH
Catalog No.:BCC2795
CAS No.:53990-33-3
- Luteolin 7,3'-di-O-glucuronide
Catalog No.:BCN5396
CAS No.:53965-08-5
- Ginsenoside F1
Catalog No.:BCN1244
CAS No.:53963-43-2
- Glycyrrhizic acid ammonium salt
Catalog No.:BCN5943
CAS No.:53956-04-0
- Aristolactam BIII
Catalog No.:BCN5718
CAS No.:53948-10-0
- Aristolactam BII
Catalog No.:BCN5717
CAS No.:53948-09-7
- Aristolactam AII
Catalog No.:BCN3924
CAS No.:53948-07-5
- Corylin
Catalog No.:BCN5716
CAS No.:53947-92-5
- Apterin
Catalog No.:BCN3910
CAS No.:53947-89-0
- Sodium salicylate
Catalog No.:BCC4846
CAS No.:54-21-7
- Furosemide
Catalog No.:BCC3782
CAS No.:54-31-9
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Idoxuridine
Catalog No.:BCC4666
CAS No.:54-42-2
- Pilocarpine HCl
Catalog No.:BCC4702
CAS No.:54-71-7
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Albendazole Oxide
Catalog No.:BCC4757
CAS No.:54029-12-8
- Etonogestrel
Catalog No.:BCC5230
CAS No.:54048-10-1
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
Biomarkers of food intake for nuts and vegetable oils: an extensive literature search.[Pubmed:30923582]
Genes Nutr. 2019 Mar 19;14:7.
Nuts and vegetable oils are important sources of fat and of a wide variety of micronutrients and phytochemicals. Following their intake, several of their constituents, as well as their derived metabolites, are found in blood circulation and in urine. As a consequence, these could be used to assess the compliance to a dietary intervention or to determine habitual intake of nuts and vegetable oils. However, before these metabolites can be widely used as biomarkers of food intake (BFIs), several characteristics have to be considered, including specificity, dose response, time response, stability, and analytical performance. We have, therefore, conducted an extensive literature search to evaluate current knowledge about potential BFIs of nuts and vegetable oils. Once identified, the strengths and weaknesses of the most promising candidate BFIs have been summarized. Results from selected studies have provided a variety of compounds mainly derived from the fatty fraction of these foods, but also other components and derived metabolites related to their nutritional composition. In particular, alpha-linolenic acid, urolithins, and 5-Hydroxyindole-3-Acetic Acid seem to be the most plausible candidate BFIs for walnuts, whereas for almonds they could be alpha-tocopherol and some catechin-derived metabolites. Similarly, several studies have reported a strong association between selenium levels and consumption of Brazil nuts. Intake of vegetable oils has been mainly assessed through the measurement of specific fatty acids in different blood fractions, such as oleic acid for olive oil, alpha-linolenic acid for flaxseed (linseed) and rapeseed (canola) oils, and linoleic acid for sunflower oil. Additionally, hydroxytyrosol and its metabolites were the most promising distinctive BFIs for (extra) virgin olive oil. However, most of these components lack sufficient specificity to serve as BFIs. Therefore, additional studies are necessary to discover new candidate BFIs, as well as to further evaluate the specificity, sensitivity, dose-response relationships, and reproducibility of these candidate biomarkers and to eventually validate them in other populations. For the discovery of new candidate BFIs, an untargeted metabolomics approach may be the most effective strategy, whereas for increasing the specificity of the evaluation of food consumption, this could be a combination of different metabolites.
Anxiolytic effect of a novel 9,10-dihydrophenanthrene, juncuenin H, is associated with metabolic changes in cortical serotonin/dopamine levels in mice.[Pubmed:30825572]
Fitoterapia. 2019 Feb 27;134:165-171.
Two novel phenanthrenoids, juncuenin H (1) and dijuncuenin B (2), together with eight known phenanthrenoids, effusol (3), dehydroeffusol (4), juncusol (5), dehydrojuncusol (6), juncuenin B (7), dehydrojuncuenin B (8), juncuenin A (9), and dehydrojuncuenin A (10), were isolated from the underground parts of Juncus setchuenensis. The structures of the compounds were determined by 1D and 2D NMR and mass spectroscopy. The anxiolytic activities of compounds 1, 6, 9, and 10 were evaluated. In order to explore the mechanisms underlying their anxiolytic activities, the levels of serotonin (5-HT), dopamine (DA), and their metabolites in the cerebral cortex and hippocampus of mice treated with compound 1 were determined by quantitative mass spectrometry. The mice treated with compound 1 had significantly lower levels of 5-HT, 3-methoxytyramine (3-MT), 5-Hydroxyindole-3-Acetic Acid (5-HIAA), homovanillic acid (HVA), and 3, 4-dihydroxyphenylacetic acid (DOPAC) in the cerebral cortex than those of the vehicle control-treated mice. The levels of HVA and 5-HIAA in the hippocampus were also significantly lower in the mice treated with compound 1 than in the control group mice. These results suggest that the metabolic changes, reflected in the levels of DA and/or 5-HT, may contribute to the anxiolytic activity of the phenanthrenoids studied herein.
[Determination of deltamethrin and its toxicity biomarkers in rabbit urine by high performance liquid chromatography-tandem mass spectrometry].[Pubmed:30136473]
Se Pu. 2018 Jun 8;36(6):523-530.
A method was developed for the determination of biomarkers related to toxicity of deltamethrin in rabbit urine by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). The target analytes in this method are as follows:deltamethrin and its two metabolites (1R-cis)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropane carboxylic acid (dibromochrysanthemic acid) and 3-phenoxybenzoic acid (3-PBA), and five toxic biomarkers, viz. serotonin hydrochloride (5-HT), 5-Hydroxyindole-3-Acetic Acid (5-HIAA), 3-nitropropionic acid (3-NPA), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and 6-methoxyguanine. Urine samples were cleaned by matrix solid-phase dispersion extraction (MSPD) with diatomite; and protein was precipitated with trichloroacetic acid; and then the sample solutions were purified with hydrophilic-lipophilic balance (HLB) solid-phase extraction cartridges. The biomarkers were analyzed with electrospray ionization (ESI) in a positive and negative switching scan mode, in which the positive scan mode was used for deltamethrin, 5-HT, 5-HIAA, 8-OHdG, and 6-methoxyguanine, and the negative scan mode was used for (1R-cis)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropane, 3-PBA, and 3-NPA. The target compounds were quantified with the external standard using matrix calibration curves. The linear regression curves of the eight target compounds were linear with correlation coefficients no less than 0.9914. The LOD and LOQ of 5-HIAA were 20 mug/L and 50 mug/L, respectively, and the LODs and LOQs of the other analytes were 0.2-5.0 mug/L and 0.5-10 mug/L, respectively. The average recoveries of the analytes spiked in rabbit urine ranged from 74.2% to 98.7% at three levels, with relative standard deviations (RSDs) no more than 12%. The method was simple, fast, accurate, sensitive, and suitable for the detection for the exposure evaluation of deltamethrin.
Simultaneous voltammetric detection of 5-hydroxyindole-3-acetic acid and 5-hydroxytryptamine using a glassy carbon electrode modified with conducting polymer and platinised carbon nanofibers.[Pubmed:30105543]
Mikrochim Acta. 2018 Aug 13;185(9):412.
The authors describe a method for simultaneous voltammetric determination of 5-hydroxytryptamine (serotonin; 5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). A glassy carbon electrode was modified with poly(pyrrole-3-carboxylic acid) and with platinised carbon nanofibers to obtain a sensor that can quantify 5-HT and 5-HIAA with detection limits of 10 nM and 20 nM, respectively. The peak currents, best measured at voltages of 170 mV and 500 mV (vs. Ag/AgCl) for 5-HT and 5-HIAA, increase linearly in the 0.01-100 muM concentration range for both analytes. The method was successfully applied to the quantitation of 5-HT and 5-HIAA in spiked artificial urine samples, and the sensor can be used up to 10 days. Graphical abstract A new electroanalytical device was developed for separation and quantitation of 5-hydroxytryptamine (5-HT) and 5-Hydroxyindole-3-Acetic Acid (5-HIAA), based on stripping square wave voltammetry, exploiting conducting polymer surfaces on platinised carbon nanofiber supports.
Monolithic nano-porous polymer in microfluidic channels for lab-chip liquid chromatography.[Pubmed:30101052]
Nano Converg. 2018;5(1):19.
In this paper, a nano-porous polymer has been integrated into the microfluidics device as on-chip monolithic liquid chromatography column for separation of chemical and biological samples. Monolithic nano-porous polymer (MNP) was formed and firmly grafted on the surface of the microfluidic channel. Neurotransmitters, 5-Hydroxyindole-3-Acetic Acid (5-HIAA) and 5-hydroxytryptamine (serotonin, 5-HT), were successfully separated with the developed on-chip MNP column.
UPLC-HRMS-Based Plasma Metabolomic Profiling of Novel Biomarkers by Treatment with KDZI in Cerebral Ischemia Reperfusion Rats.[Pubmed:29849010]
Molecules. 2018 May 30;23(6). pii: molecules23061315.
Kudiezi injection (KDZI), also known as Diemailing injection, is a traditional Chinese medicine injection of the composite plant Ixeris sonchifolia Hance (also known as Kudiezi), and has been widely used to treat coronary heart disease, angina pectoris, and cerebral infarction, but its pharmacological mechanisms remain unclear. This study is designed to explore the effects of KDZI on middle cerebral artery occlusion and reperfusion (MCAO/R) rats, and to identify metabolic features of cerebral ischemia reperfusion by using a nontargeted metabolic profiling method based on ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS). In this process, 32 potential biomarkers were found in plasma. KDZI significantly upregulated the levels of taurochenodesoxycholic acid, leucine, l-phenylalanine, l-tryptophan, arachidonic acid (ARA), and phosphatidyl ethanolamines (PE), phosphatidyl cholines (PC) and downregulated the levels of l-valine and 5-Hydroxyindole-3-Acetic Acid (5-HIAA) in plasma. The results indicated that the mechanisms of KDZI on MCAO/R were related to the mechanisms of amino acid and lipid metabolism.
Monoaminergic and aminoacidergic receptors are involved in the antidepressant-like effect of ginsenoside Rb1 in mouse hippocampus (CA3) and prefrontal cortex.[Pubmed:29802841]
Brain Res. 2018 Nov 15;1699:44-53.
Ginsenoside Rb1 (Rb1), as the major bioactive ingredient of Panax ginseng C.A. Meyer, elicited a novel antidepressant-like effect in the forced swim test (FST) in chronic unpredictable mild stress (CUMS) rats in our previous study. To further explore the molecular mechanism of Rb1 on the neurotransmitters such as 5-hydroxytryptamine (5-HT), 5-Hydroxyindole-3-Acetic Acid (5-HIAA), norepinephrine (NE), dopamine (DA), homovanillic acid (HVA), dihydroxyphenylacetic acid (DOPAC), glutamate (Glu) and gamma-aminobutyric acid (GABA) in this antidepressant-like effect, the neurochemical changes in the monoaminergic and aminoacidergic receptors were investigated in the present pharmacological study by using reuptake inhibitors, receptors agonists and antagonists. The results showed that a sub-effective dose of Rb1 (5mg/kg, p.o.) co-administered with fluoxetine (1mg/kg, i.p., a selective serotonin reuptake inhibitor), reboxetine (2.5mg/kg, i.p., a noradrenalin reuptake inhibitor), bupropion (10mg/kg, i.p., a dopaminergic reuptake inhibitor), Mk-801 (0.05mg/kg, i.p., an N-methyl-d-aspartic acid (NMDA) receptor antagonist) or baclofen (0.1mg/kg, i.p., a selective GABA agonist) significantly decreased the immobility time in the FST. In addition, pretreating mice with NAN190 (0.5mg/kg, i.p., a 5-HT1A receptor antagonist), ketanserin (5mg/kg, i.p., a 5-HT2A/2C receptor antagonist), ondansetron (1mg/kg, i.p., a 5-HT3A receptor antagonist), prazosin (1mg/kg, i.p., an alpha1-adrenoceptor antagonist), yohimbine (1mg/kg, i.p., an alpha2-adrenoceptor antagonist), SCH23390 (0.05mg/kg, i.p., a selective D1 receptor antagonist), haloperidol (0.2mg/kg, i.p., a non-selective D2 receptor antagonist), NMDA (75mg/kg, i.p., an agonist at the glutamate site) or bicuculline (4mg/kg, i.p., a competitive GABA antagonist) reversed the antidepressant-like effect of Rb1 (10mg/kg, p.o.) in the FST. The results obtained for the neurotransmitters in the mouse hippocampus (CA3) and prefrontal cortex showed that Rb1 up-regulated the levels of 5-HT, 5-HIAA, NE, DA, and GABA and decreased the level of Glu. However, there were no significant differences in HVA or DOPAC. Furthermore, there were no significant alterations in the total path of spontaneous locomotor activity in all treatments. These results suggest that both monoaminergic (serotonergic, noradrenergic and dopaminergic) and aminoacidergic (glutamatergic and GABAergic) receptors may be involved in the antidepressant-like effect of Rb1.
Central serotonin attenuates LPS-induced systemic inflammation.[Pubmed:28723348]
Brain Behav Immun. 2017 Nov;66:372-381.
Serotonin (5-HT) is a neuromodulator involved in several central-mediated mechanisms, such as endocrine processes, behavior, and sleep. Dysfunction of the serotonergic system is mainly linked to psychiatric disorders, but emerging evidence suggests that immune system activation may also alter brain 5-HT signaling. However, whether central 5-HT modulates systemic inflammation (SI) remains unknown. For this purpose, male Wistar rats (280-350g, 8-9weeks) were submitted to the experimental protocols beginning between 9 and 10AM with the performance of injections. The animals were housed at controlled conditions [temperature (25+/-1 degrees C), light (06:00-18:00) and humidity (60-65%)]. Thus, we measured 5-HT and its metabolite 5-Hydroxyindole-3-Acetic Acid (5-HIAA) in the anteroventral preoptic region [(AVPO) - the hierarchically most important region for body temperature (Tb) control] during lipopolysaccharide (LPS)-induced SI. We also combined LPS (100mug/kg) treatment with intracerebroventricular (icv) injection of 5-HT (5, 10 and 40mug/muL) and measured Tb ("hallmark" of SI), AVPO prostaglandin E2 [(PGE2) - an essential mediator of fever] and prostaglandin D2 [(PGD2) - a cryogenic mediator], plasma corticosterone [(CORT) - a stress marker with an endogenous anti-inflammatory effect] and interleukin-6 [(IL-6) - an immune mediator] levels. Detection limits of PGE2, PGD2, CORT and IL-6 assays were 39.1-2500pg/mL, 19.5-2500pg/mL, 0.12-2000mug/dL, and 0.125-8ng/mL, respectively. We also assessed tail skin temperature [used to calculate heat loss index (HLI)] to assess a key thermoeffector mechanism. As expected we observed LPS-induced increases in Tb, AVPO PGE2 (whereas PGD2 remained unchanged), plasma CORT and IL-6 levels, as well as a decrease in HLI. These changes were accompanied by reduced levels of AVPO 5-HT and 5-HIAA. Furthermore, we also observed a negative correlation between 5-HT and plasma CORT levels. Moreover, icv 5-HT (5, 10 and 40mug/muL) microinjection caused a U-shaped dose-response curve in LPS fever, in which the intermediate dose reduced the febrile response. Icv 5-HT (10mug/muL) microinjection prevented the LPS-induced increases in AVPO PGE2 (whereas not altering PGD2), plasma CORT and IL-6 levels, as well as preventing reduced HLI. Our data are consistent with the notion that AVPO 5-HT synthesis is down-regulated during SI, favoring AVPO PGE2 synthesis and consequently potentiating the immune response. These results reveal a novel effect of central 5-HT as an anti-inflammatory neuromodulator that may take place during psychiatric disorder treatment with 5-HT reuptake inhibitors as well as suggesting that 5-HT modulation per se is a potential therapeutic approach for inflammatory diseases.
Disturbed tryptophan metabolism correlating to progression and metastasis of esophageal squamous cell carcinoma.[Pubmed:28342863]
Biochem Biophys Res Commun. 2017 May 6;486(3):781-787.
Esophageal squamous cell carcinoma (ESCC) is one of the most frequent malignancies worldwide. Lymph node metastasis is the leading cause of death in ESCC patients. To identify early diagnostic and prognostic biomarkers of ESCC and elucidate underlying pathogenesis of the disease, a targeted metabolomics strategy based on liquid chromatography combined with tandem mass spectrometry was applied to explore tryptophan metabolism between ESCC patients, metastatic ESCC patients (mESCC), and healthy controls. Statistical analysis on metabolite expression abundance and compound concentration ratio was conducted to discriminate patients from healthy controls. The concentration ratio of kynurenine, 5-hydroxytryptophan, 5-Hydroxyindole-3-Acetic Acid, 5-hydroxytryptamine to their precursor tryptophan were identified as potential biomarkers, presenting high diagnostic capacity for distinguishing ESCC and mESCC patients from healthy controls. Moreover, a prognostic prediction model was also built on these ratios to distinguish metastasis patients from non-metastasis patients successfully. The high performance of ESCC prediction models suggest that concentration ratios of compounds may be used as biomarkers for early diagnosis and prognosis of the disease. In addition, concentration ratios of compounds show a progressively increased trend from non-metastasis to metastasis patients compared with healthy controls, which is in accordance with process of malignant transformation of ESCC. This interested finding suggests that disturbed tryptophan metabolism is correlated to progression and metastasis of ESCC since concentration ratios of compounds reflect activity of enzymes involved in tryptophan metabolism. This study reveals the impact of tryptophan metabolism to tumorigenesis and metastasis of ESCC, which help biologists investigate mechanism of the disease.
Long-term effects of pre-pubertal fluoxetine on behaviour and monoaminergic stress response in stress-sensitive rats.[Pubmed:27819195]
Acta Neuropsychiatr. 2017 Aug;29(4):222-235.
OBJECTIVE: Although prescription rates of antidepressants for children and adolescents have increased, concerns have been raised regarding effects on neurodevelopment and long-term outcome. Using a genetic animal model of depression, this study investigated the long-term effects of pre-pubertal administration of fluoxetine (FLX) on depressive-like behaviour in early adulthood, as well as on central monoaminergic response to an acute stressor. We postulated that pre-pubertal FLX will have lasting effects on animal behaviour and monoaminergic stress responses in early adulthood. METHODS: Flinders sensitive line (FSL) rats received 10 mg/kg/day FLX subcutaneously from postnatal day 21 (PnD21) to PnD34 (pre-pubertal). Thereafter, following normal housing, rats were either subjected to locomotor testing and the forced swim test (FST) on PnD60 (early adulthood), or underwent surgery for microdialysis, followed on PnD60 by exposure to acute swim stress and measurement of stressor-induced changes in plasma corticosterone and pre-frontal cortical monoamine concentrations. RESULTS: Pre-pubertal FLX did not induce a late emergent effect on immobility in FSL rats on PnD60, whereas locomotor activity was significantly decreased. Acute swim stress on PnD60 significantly increased plasma corticosterone levels, and increased pre-frontal cortical norepinephrine (NE) and 5-Hydroxyindole-3-Acetic Acid (5-HIAA) concentrations. Pre-pubertal FLX significantly blunted the pre-frontal cortical NE and 5-HIAA response following swim stress on PnD60. Baseline dopamine levels were significantly enhanced by pre-pubertal FLX, but no further changes were induced by swim stress. CONCLUSION: Pre-pubertal FLX did not have lasting antidepressant-like behavioural effects in genetically susceptible, stress-sensitive FSL rats. However, such treatment reduced locomotor activity, abrogated noradrenergic and serotonergic stressor responses and elevated dopaminergic baseline levels in adulthood.
Ultrahigh-Performance Liquid Chromatography (UHPLC)-Tandem Mass Spectrometry (MS/MS) Quantification of Nine Target Indoles in Sparkling Wines.[Pubmed:27148823]
J Agric Food Chem. 2016 Jun 15;64(23):4772-6.
An ultrahigh-performance liquid chromatography (UHPLC)-tandem mass spectrometry (MS/MS) method was developed for the simultaneous determination of nine target indoles in sparkling wines. The proposed method requires minimal sample pretreatment, and its performance parameters (accuracy, repeatability, LOD, and matrix effect) indicate that it is suitable for routine analysis. Four indoles were found at detectable levels in commercial Cava samples: 5-methoxytryptophol (5MTL), tryptophan (TRP), tryptophan ethyl ester (TEE), and N-acetylserotonin (NSER). Two of them, NSER and 5MTL, are reported here for the first time in sparkling wines, with values of 0.3-2 and 0.29-29.2 mug/L, respectively. In the same samples, the contents of melatonin (MEL), serotonin (SER), 5-hydroxytryptophan (5-OHTRP), 5-Hydroxyindole-3-Acetic Acid (5OHIA), and 5-methoxy-3-indoleacetic acid (5MIA) were all below the corresponding limits of detection.
Cerebral Metabolic Profiling of Hypothermic Circulatory Arrest with and Without Antegrade Selective Cerebral Perfusion: Evidence from Nontargeted Tissue Metabolomics in a Rabbit Model.[Pubmed:26960374]
Chin Med J (Engl). 2016 Mar 20;129(6):702-8.
BACKGROUND: Antegrade selective cerebral perfusion (ASCP) is regarded to perform cerebral protection during the thoracic aorta surgery as an adjunctive technique to deep hypothermic circulatory arrest (DHCA). However, brain metabolism profile after ASCP has not been systematically investigated by metabolomics technology. METHODS: To clarify the metabolomics profiling of ASCP, 12 New Zealand white rabbits were randomly assigned into 60 min DHCA with (DHCA+ASCP [DA] group, n = 6) and without ( DHCA [D] group, n = 6) ASCP according to the random number table. ASCP was conducted by cannulation on the right subclavian artery and cross-clamping of the innominate artery. Rabbits were sacrificed 60 min after weaning off cardiopulmonary bypass. The metabolic features of the cerebral cortex were analyzed by a nontargeted metabolic profiling strategy based on gas chromatography-mass spectrometry. Variable importance projection values exceeding 1.0 were selected as potentially changed metabolites, and then Student's t-test was applied to test for statistical significance between the two groups. RESULTS: Metabolic profiling of brain was distinctive significantly between the two groups (Q 2 Y = 0.88 for partial least squares-DA model). In comparing to group D, 62 definable metabolites were varied significantly after ASCP, which were mainly related to amino acid metabolism, carbohydrate metabolism, and lipid metabolism. Kyoto Encyclopedia of Genes and Genomes analysis revealed that metabolic pathways after DHCA with ASCP were mainly involved in the activated glycolytic pathway, subdued anaerobic metabolism, and oxidative stress. In addition, L-kynurenine (P = 0.0019), 5-methoxyindole-3-acetic acid (P = 0.0499), and 5-Hydroxyindole-3-Acetic Acid (P = 0.0495) in tryptophan metabolism pathways were decreased, and citrulline (P = 0.0158) in urea cycle was increased in group DA comparing to group D. CONCLUSIONS: The present study applied metabolomics analysis to identify the cerebral metabolic profiling in rabbits with ASCP, and the results may shed new lights that cerebral metabolism is better preserved by ASCP compared with DHCA alone.
Hydrophilic interaction chromatography combined with dispersive liquid-liquid microextraction as a preconcentration tool for the simultaneous determination of the panel of underivatized neurotransmitters in human urine samples.[Pubmed:26747692]
J Chromatogr A. 2016 Jan 29;1431:111-121.
A simple and sensitive method using dispersive liquid-liquid microextraction (DLLME) followed by liquid chromatography coupled to mass spectrometry (LC-MS) with a hydrophilic interaction chromatography (HILIC) column was developed for the simultaneous determination of 13 compounds of different polarities, comprising monoamine neurotransmitters (dopamine, norepinephrine, epinephrine and serotonin) along with their respective precursors and metabolites, in human urine samples. The microextraction procedure was based on the fast injection of a mixture of ethanol (disperser solvent) and dichloromethane (extraction solvent) into a human urine sample, forming a cloudy solution in the Eppendorf tube. After centrifugation, the sedimented phase was collected and subsequently analyzed by LC-HILIC-MS in about 12min without a derivatization step. The separation was performed on an XBridge Amide BEH column 3.0x100mm, 3.5mm and the mobile phase consisted of phase A: 10mM ammonium formate buffer in water pH 3.0 and phase B: 10 mM ammonium formate buffer in acetonitrile, under gradient program elution. Tyrosine, tryptophan, 5-hydroxytryptophan, dopamine, epinephrine, norepinephrine, serotonin, 3-methoxytyramine, 5-Hydroxyindole-3-Acetic Acid, 3,4-dihydroxy-l-phenylalanine and norvaline (internal standard) were detected in the positive ionization mode. While vanillylmandelic acid, homovanillic acid, 3,4-dihydroxyphenylacetic acid and 3,4-dihydroxybenzylamine (internal standard) were detected in the negative ionization mode. Parameters influencing DLLME and LC-HILIC-MS were investigated. Under the optimum conditions, the proposed method exhibited a low detection limit (5-10ngmL(-1)), and good linearity with R between 0.9991 and 0.9998. The recoveries in human urine samples were 99.0%+/-3.6%. for the 13 studied biogenic amines with intra- and inter-day RSDs of 0.24-9.55% and 0.31-10.0%, respectively. The developed DLLME-LC-MS method could be successfully applied for the determination of trace amounts of polar endogenous compounds, such as neurotransmitters, in human urine samples, including samples with a reduced volume obtained from pediatric patients.


