(+)-CamphorCAS# 464-49-3 |
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- Camphor
Catalog No.:BCN8297
CAS No.:76-22-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 464-49-3 | SDF | Download SDF |
| PubChem ID | 230921 | Appearance | Oil |
| Formula | C10H16O | M.Wt | 152.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | D-(+)-Camphor; (1R)-(+)-Camphor | ||
| Solubility | DMSO : ≥ 50 mg/mL (328.45 mM) *"≥" means soluble, but saturation unknown. | ||
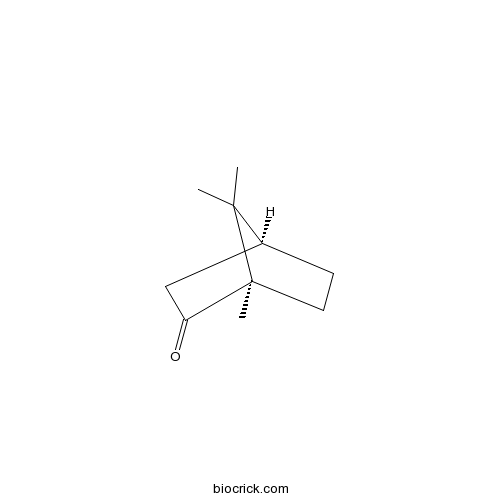
| Chemical Name | (1S,4R)-4,7,7-trimethylbicyclo[2.2.1]heptan-3-one | ||
| SMILES | CC1(C2CCC1(C(=O)C2)C)C | ||
| Standard InChIKey | DSSYKIVIOFKYAU-XVKPBYJWSA-N | ||
| Standard InChI | InChI=1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7-,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Camphor is highly repellent to the beetles with overall repellency in the range of 80 - 100%. |

(+)-Camphor Dilution Calculator

(+)-Camphor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5703 mL | 32.8515 mL | 65.703 mL | 131.406 mL | 164.2576 mL |
| 5 mM | 1.3141 mL | 6.5703 mL | 13.1406 mL | 26.2812 mL | 32.8515 mL |
| 10 mM | 0.657 mL | 3.2852 mL | 6.5703 mL | 13.1406 mL | 16.4258 mL |
| 50 mM | 0.1314 mL | 0.657 mL | 1.3141 mL | 2.6281 mL | 3.2852 mL |
| 100 mM | 0.0657 mL | 0.3285 mL | 0.657 mL | 1.3141 mL | 1.6426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(+)-Camphor is an ingredient in cooking, and as an embalming fluid for medicinal purposes,
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- Ko 143
Catalog No.:BCC1684
CAS No.:461054-93-3
- Eact
Catalog No.:BCC6313
CAS No.:461000-66-8
- Larixyl acetate
Catalog No.:BCC8195
CAS No.:4608-49-5
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Quinamine
Catalog No.:BCN6590
CAS No.:464-85-7
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
Simultaneous analysis of volatile and semi-volatile components in a topical formulation by gas chromatography using a programmed temperature vaporization inlet and flame ionization detection.[Pubmed:30974410]
J Pharm Biomed Anal. 2019 Apr 1;171:65-72.
Topical formulations are medications applied locally on the skin to treat ailment. They are made up of complex mixtures of active ingredients and excipients. Till date, no analytical method has been found in literature that is able to simultaneously analyze volatile and semi-volatile actives present in topical formulations. In this work, an analytical procedure by gas chromatography equipped with a programmed temperature vaporizing (PTV) inlet and a flame ionization detector was developed and validated for the simultaneous quantitative determination of volatile and semi-volatile actives such as (+)-Camphor, L-menthol, methyl salicylate, ethyl salicylate, salicylic acid, glycol monosalicylate and capsaicin in a topical formulation. Liquid-liquid extraction was used to isolate the components of interest prior to injection into the gas chromatographic system. All target analytes were completely separated from each other and a linear calibration curve was achieved for all analytes with a determination coefficient > 0.995. 2-phenoxyethanol was used as internal standard for quantitation. Good repeatability and recovery values were achieved and reported. This method reports for the first time, the simultaneous quantitative analysis of volatile and semi-volatile active pharmaceutical ingredients in a single measurement. The developed method was successfully applied to the analysis of real pharmaceutical samples and the described analytical protocols can be recommended for routine analysis of both volatile and semi-volatile actives in the topical formulation.
Real-Life Experience with Selexipag as an Add-On Therapy to Oral Combination Therapy in Patients with Pulmonary Arterial or Distal Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis.[Pubmed:30963265]
Lung. 2019 Apr 8. pii: 10.1007/s00408-019-00222-7.
BACKGROUND: Patients with pulmonary arterial hypertension (PAH) and distal chronic thromboembolic pulmonary hypertension (CTEPH) who still reveal risk factors of worse prognosis on double combination therapy may benefit from add-on therapy with the novel oral selective prostacyclin receptor agonist selexipag. METHODS: We reviewed all patients with PAH/distal CTEPH in the Zurich cohort who received selexipag as add-on to oral combination therapy and retrieved New York Heart Association (NYHA) functional class, 6-min walk distance (6MWD), NT-pro-BNP, quality of life questionnaires (CAMPHOR and EuroQoL), tricuspid pressure gradient (TPG) by echocardiography and cardiopulmonary exercise test parameters (power output and oxygen uptake). RESULTS: Twenty-three patients with PAH/CTEPH (20/3), 14 females, median (quartiles) age 56 (46; 66) years received an oral triple therapy containing selexipag at a median dose of 2000 (1600; 3100) mcg during 221 (113; 359) days. The following parameters were stabilized from baseline to last FU: 6MWD (440 (420; 490) to 464 (420; 526) m), NYHA class (three to two), NT-pro-BNP (326 (167; 1725) to 568 (135; 1856) ng/l), TPG, power output, and oxygen uptake. Quality of life reflected by the (+)-Camphor and EuroQoL improved. CONCLUSIONS: Early initiation of triple oral combination therapy including selexipag in PAH/CTEPH with intermediate risk factor profile may help to stabilize functional class, exercise performance, and pulmonary hemodynamics in a real-life setting and potentially improves quality of life. Whether these beneficial effects can be truly attributed to the addition of selexipag should be addressed in future randomized controlled trials.
Effect of simultaneous snail slime-aided degradation and yeast fermentation on terpenoid composition of plantain pseudostem waste.[Pubmed:30961480]
Curr Pharm Biotechnol. 2019 Apr 8. pii: CPB-EPUB-97888.
BACKGROUND: In this study, local sustainable enzyme sources involving excised digestive juice of African land snail and yeast were to achieve the simultaneous saccharification and fermentation (SSF) of plantain pseudo stem (PPS) waste, and afterwards examined their effects on terpenoids using gas chromatograpy coupled to a fluorescent ionization detector (GC-FID). RESULTS: The most abundant terpenoids were found in the order alpha-pinene > borneol > (+)-Camphor > humulene > beta-caryophellene, while the least in abundance were cis ocimene (8.78x10-6 mg/100g), and cyperene (1.81x10-5 mg/100g). The application of exclusive fermentation, and SSF respectively elevated azuluene by 95.46 and 99.6 %, while pinene-2-ol was elevated by 83.02 and 98.57 % respectively. Both exclusive fermentation and SSF had no effect on myrcene, cyperene, ethyl cinnamate, germacrene b, valencene, beta selinene, aromadendrene, and taraxerol, while the degree of degradation of some of the terpenoids by both processes were respectively as follows; gama muurolene (100%), beta-caryophyllene (97.60 and 93.14 %), alpha-terpinenyl acetate (91.95 and 83.16 %), geranyl acetate (94.81 and 43.87 %), and terpinen-4-ol (94.40 and 57.00 %). CONCLUSION: The findings of this study indicate the imminent application of simultaneous saccharification and fermentation for the enhancement of bioactivities of terpenoids.
Comparative volatile composition, antioxidant and cytotoxic evaluation of the essential oil of Zhumeria majdae from south of Iran.[Pubmed:30944712]
Iran J Basic Med Sci. 2019 Jan;22(1):80-85.
Objectives: The purpose of this study was to evaluate variations in yields, volatile composition and biological activities of essential oils (EOs) obtained from the aerial parts of Zhumeria majdae collected from five localities of the south of Iran. Materials and Methods: The EOs were analyzed using gas chromatography and gas chromatography-mass spectrometry techniques. The antioxidant activity of the EOs was tested using DPPH and beta-carotene/linoleic acid assays. In vitro cytotoxicity was tested against two cancer cell lines (A375 and MCF7) using MTT assay. Results: The oils yield varied from 6.3% (S2) to 10.2% (V/W) (S4). All of five investigated EOs samples presented three major compounds: linalool (24.4-34.6%), (+)-Camphor (26.1-34.7%) and trans-linalool oxide (7.6-28.6%). Although the main constituents were common, their percentages were different. Among samples, S1 had a better antioxidant activity in both DPPH and beta-carotene/linoleic acid methods (IC50= 8.01 and 11.77 mg/ml, respectively). In vitro cytotoxicity against two cancer cell lines of human melanoma cell line (A375) and breast cancer cell line (MCF7), showed a moderate cytotoxicity of S3 against A375 cells with IC50 value of 624 mug/ml. Conclusion: Tangezagh (S4) plant materials revealed the highest level of oil yield as the region is recommended for collecting the plant samples.Taken together, despite the weak antioxidant and moderate cytotoxic activities of tested EOs, this study suggested a proper potential for possible use of the EOs of Z. majdae for pharmaceutical and perfume industries.
Rotational motion of a camphor disk in a circular region.[Pubmed:30934219]
Phys Rev E. 2019 Feb;99(2-1):022211.
In a two-dimensional axisymmetric system, the system symmetry allows rotational or oscillatory motion as stable stationary motion for a symmetric self-propelled particle. In the present paper, we studied the motion of a (+)-Camphor disk confined in a two-dimensional circular region. By reducing the mathematical model describing the dynamics of the motion of a (+)-Camphor disk and the concentration field of (+)-Camphor molecules on a water surface, we analyzed the reduced equations around a bifurcation point where the rest state at the center of the system becomes unstable. As a result, we found that rotational motion is stably realized through the double-Hopf bifurcation from the rest state. The theoretical results were confirmed by numerical calculation and corresponded well to the experimental results.
Traffic-related dustfall and NOx, but not NH3, seriously affect nitrogen isotopic compositions in soil and plant tissues near the roadside.[Pubmed:30933763]
Environ Pollut. 2019 Mar 25;249:655-665.
Ammonia (NH3) emissions from traffic have received particular attention in recent years because of their important contributions to the growth of secondary aerosols and the negative effects on urban air quality. However, few studies have been performed on the impacts of traffic NH3 emissions on adjacent soil and plants. Moreover, doubt remains over whether dry nitrogen (N) deposition still contributes a minor proportion of plant N nutrition compared with wet N deposition in urban road environments. This study investigated the delta(15)N values of road dustfall, soil, moss, (+)-Camphor leaf and (+)-Camphor bark samples collected along a distance gradient from the road, suggesting that samples collected near the road have significantly more positive delta(15)N values than those of remote sites. According to the SIAR model (Stable Isotope Analysis in R) applied to dustfall and moss samples from the roadside, it was found that NH3 from traffic exhaust (8.8+/-7.1%) contributed much less than traffic-derived NO2 (52.2+/-10.0%) and soil N (39.0+/-13.8%) to dustfall bulk N; additionally, 68.6% and 31.4% of N in mosses near the roadside could be explained by dry N deposition (only 20.4+/-12.5% for traffic-derived NH3) and wet N deposition, respectively. A two-member mixing model was used to analyse the delta(15)N in continuously collected mature (+)-Camphor leaf and (+)-Camphor bark samples, which revealed a similarity of the delta(15)N values of plant-available deposited N to (15)N-enriched traffic-derived NOx-N. We concluded that a relatively high proportion of N inputs in urban road environments was contributed by traffic-related dustfall and NOx rather than NH3. These information provide useful insights into reducing the impacts of traffic exhaust on adjacent ecosystems and can assist policy makers in determining the reconstruction of a monitoring network for N deposition that reaches the road level.
Oxidative stress-mediated apoptotic cell death induced by camphor in sod1-deficient Schizosaccharomyces pombe.[Pubmed:30931102]
Toxicol Res (Camb). 2018 Nov 27;8(2):216-226.
Camphor is one of the monoterpenes widely used in cosmetics, pharmaceutics and the food industry. In this study, we aimed to assess the oxidative, cytotoxic and apoptotic effects of (+)-Camphor on the fission yeast (Schizosaccharomyces pombe), which is a promising unicellular model organism in mechanistic toxicology and cell biology. Since Sod1 is the main radical scavenger in the cell, we used sod1 mutants to understand whether (+)-Camphor-induced ROS accumulation caused higher cytotoxicity and apoptosis. (+)-Camphor exposure (0-2000 mg L(-1)) caused significant cytotoxicity in yeast, particularly in sod1Delta cells. DCFDA (2,7-dichlorodihydrofluorescein diacetate) fluorescence and NBT (p-nitro-blue tetrazolium chloride) reduction increased (at least 2.5-3-fold in sod1Delta cells) in correlation with (+)-Camphor concentrations (800-1200 mg L(-1)), showing higher ROS levels and oxidative stress. Moreover, cells, stained with acridine orange/ethidium bromide, showed an apoptotic morphology with nuclear fragmentation and condensation. DAPI (4',6-diamidino-2-phenylindole) staining was used to validate the apoptotic nuclear morphology. Dramatically increased mitochondrial impairment, which was higher in sod1Delta cells than in wild type cells, was shown by rhodamine 123 staining. In conclusion, (+)-Camphor-induced excessive ROS production, which could not be prevented significantly in sod1 mutants, caused a dramatic increase in mortality rates due to intrinsic apoptosis revealed by mitochondrial impairment and apoptotic nuclear morphology. The potential effects of (+)-Camphor on apoptotic cell death and the underlying mechanisms were clarified in the unicellular eukaryotic model, S. pombe.
Ecotoxicity of a new biopesticide produced by Lavandula luisieri on non-target soil organisms from different trophic levels.[Pubmed:30927731]
Sci Total Environ. 2019 Mar 24;671:83-93.
Plant-based biopesticides have become an eco-friendly alternative to synthetic pesticides by reducing the undesired environmental impacts and side-effects on human health. However, their effects on the environment and especially on non-target organisms have been little studied. This study analyses the ecotoxicological effects of the extract of Lavandula luisieri on soil non-target organisms from different trophic levels: the earthworm Eisenia fetida, the plant Allium cepa and a natural-soil microbial community whose taxonomy was analysed through 16S rRNA gene sequencing. The extract tested is the hydrolate -product from a semi industrial steam distillation process- of a Spanish pre-domesticated variety of L. luisieri. This hydrolate has been recently shown to have bionematicide activity against the root-knot nematode Meloidogyne javanica. A previous study showed that the main components of the hydrolate are (+)-Camphor and 2,3,4,4-Tetramethyl-5-methylidenecyclopent-2-en-1-one. Hydrolate caused acute toxicity (LC50 2.2% v/v) on A. cepa, while only a slight toxicity on E. fetida (LC50>0.4mL/g). All the concentrations tested (from 1 to 100% v/v) caused a significant decrease in bacterial growth (LC50 9.8% v/v after 120h of exposure). The physiological diversity of the community was also significantly altered, except in the case of the lowest concentration of hydrolate (1% v/v). The ability of soil microbial communities to use a variety of carbon sources increased for all substrates at the highest concentrations. These results show that both the plants and bacterial communities of the soil can be affected by the application of biopesticides based on these hydrolates, which highlights the need for a more detailed risk assessment during the development of plant-based products.
A comparative study on chemical composition and antimicrobial activity of essential oils from three Phlomis species from Uzbekistan.[Pubmed:30919659]
Nat Prod Res. 2019 Mar 28:1-6.
Essential oils obtained from the aerial parts of Phlomis bucharica, P. salicifolia and P. sewerzowii were determined using GC-FID and GC-MS methods. A total of 76 components were identified in the three species representing 97.12, 88.34, and 96.41% of the whole oil, respectively. High percentages of thymol (20.41%) and (+)-Camphor (14.46%) exist in P. bucharica oil. Methyl palmitate predominates in P. salicifolia oil representing 51.15% whereas thymol (35.76%) is the major constituent in P. sewerzowii essential oil. GC-MS analyses showed that P. bucharica and P. sewerzowii are more closely related comparable to P. salicifolia. The antimicrobial activity of the essential oils was assessed against different microorganisms using agar-disc diffusion and broth microdilution assay. Among the three tested species, the essential oil of P. salicifolia showed the highest antibacterial activity.
Virtual structural similarity elucidates bioactivity of Fenchone: Enriched phytochemical in fennel essential oil.[Pubmed:30907324]
Curr Drug Discov Technol. 2019 Mar 21. pii: CDDT-EPUB-97500.
BACKGROUND: Fenchone is a natural monoterpene abundantly present in Fennel essential oil. It is known for its wound healing properties but its other biological activities are less explored. METHODS: We use an in silico structural similarity searching approaches to identify various biological activities of Fenchone. The identified biological activities of Fenchone (purchased from Sigma Aldrich) were validated by conducting DPPH free radical scavenging assay, MTT assay, well diffusion assay for antimicrobial activity and enzymatic assays to analyze the activity of different antioxidant enzymes. RESULTS: (+)-Camphor was found to possess maximum structural similarity with Fenchone (similarity-index 100). Molecular docking demonstrates that binding modes of the Fenchone were also similar to (+)-Camphor against protein Cytochrome CYP101D1 (PDB ID: 4C9K). Fenchone was also demonstrated to possess antioxidant activity (IC50: 3.32+/-0.008mM), antimicrobial activity (MIC: 0.49mM) and a very strong antifungal activity. Fenchone protects yeast cells from H2O2 induced cytotoxicity and cytotoxic to cancerous Hela cells (IC50: 12.63+/-0.12 microM). Fenchone treatment also showed decreased activity of antioxidant enzymes Glutathione-S-transferase, Catalase and Lipid Peroxidase. CONCLUSION: To best of our knowledge this is first report which use structural similarity searching to explore biological activities of Fenchone.
Oil boundary approach for sublimation enabled camphor mediated graphene transfer.[Pubmed:30901688]
J Colloid Interface Sci. 2019 Mar 16;546:11-19.
HYPOTHESIS: Transfer of chemical-vapor-deposition (CVD) grown monolayer graphene from one substrate to another requires a transfer agent. The transfer agent usually needs to be removed by washing with organic solvent such as acetone or high temperature annealing, which is harmful to the structure integrity and intrinsic property of a graphene film. Here, we propose the use of (+)-Camphor as a transfer agent to transfer monolayer graphene onto a target dielectric substrate, which bypasses these demanding steps and only needs the common alcohol solvent rinsing. EXPERIMENTS: To facilitate a crack-free graphene transfer, the proposed approach allows the (+)-Camphor supported polycrystalline graphene to be rationally fastened with a thickened and solidified edge bead (i.e. (+)-Camphor oil-filled boundary). A layer of (+)-Camphor was first deposited onto a graphene/copper surface. The backside copper substrate was then etched away, whilst the (+)-Camphor/graphene bilayer was placed onto a SiO2/Si substrate. Finally, the (+)-Camphor remaining on the (+)-Camphor/graphene/SiO2/Si sublimed into a vapor. The graphene/SiO2 stack was then examined by microscopic, spectral and electrical characterization. FINDINGS: The results of our examination suggest that the proposed method can guarantee a clean and damage-free graphene transfer. This method is particularly attractive in the application area for nano/micro-electronics, where it provides CVD-grown graphene the ability to be used on wide varieties of substrates that are sensitive to organic solvents and high temperature.
Simultaneous GC-FID Quantification of Main Components of Rosmarinus officinalis L. and Lavandula dentata Essential Oils in Polymeric Nanocapsules for Antioxidant Application.[Pubmed:30881726]
J Anal Methods Chem. 2019 Feb 10;2019:2837406.
The essential oils (EO) of R. officinalis and L. dentata have been widely used due to their antioxidant activity. However, due to their high volatility, the loading of EO into polymeric nanocapsules (NC) represents an efficient way of retaining their effect in future topical administration. In this way, the quantitative determination of EO incorporated into NC is necessary for simultaneous monitoring of the main components of the EO during the nanoencapsulation process as well as for precise and exact dosing of the components used during the performance of in vitro and in vivo biological tests. In this study, EO were isolated by hydrodistillation in a Clevenger-type apparatus and characterized by GC-MS and GC-FID analyses. The major constituents of EO-R. officinalis were (+)-Camphor (39.46%) and 1,8-cineole (14.63%), and for EO-L. dentata were 1,8-cineole (68.59%) and beta-pinene (11.53%). A new analytical method based on GC-FID for quantification of free and encapsulated EO was developed and validated according to ICH. Linearity, limit of detection and quantification, and intra- and interday precision parameters were determined. The methods were linear and precise for the quantification of the main components of EO. The EO were encapsulated by nanoprecipitation and were analyzed by the GC-FID method validated for their direct quantification. The NC size was 200 nm with homogeneous size distribution. The quantification of the incorporated EO within a NC is an important step in NC characterization. In this way, an encapsulation efficiency of at least 59.03% and 41.15% of total EO-R. officinalis and EO-L. dentata, respectively, was obtained. Simple, repeatable, and reproducible methods were developed as an analytical tool for the simultaneous quantification of the main components of EO loaded in polymeric nanocapsules as well as their monitoring in biological assays.
Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L.[Pubmed:30875445]
Chem Biodivers. 2019 Mar 15.
The genus Euphorbia attracted the attention of many researchers worldwide, from natural products, bioactivity, and ecological perspective. The essential oils (EOs) of Euphorbia heterophylla are poorly studied. Therefore the present study aimed to provide a detailed profile of the E. heterophylla EOs as well as determine thier antioxidant and allelopathic activities. The EOs from aerial parts of E. heterophylla were extracted using hydrodistillation and analyzed via GC-MS. The antioxidant activity was determined based on scavenging of the free radicle, 1,1-Diphenyl-2-picrylhydrazyl and H2O2. Various concentrations of the EOs were tested against the noxious weed, Cenchrus echinatus. Thirty-five compounds were identified representing 100% of the total mass. Four classes of components were characterized among which terpenoids were the main components (88.70%). Monoterpenes represented the main class (69.48%), followed by sesquiterpenes (18.63%), and only one diterpenoid, kaur-16-ene, was identified. The 1,8-cineole (32.03%), (+)-Camphor (16.54%), beta-elemene (5.92%), endo-borneol (4.94%), limonene (4.27%), n-pentatriacontane (3.91%), alpha-pinene (3.89%) were the major compounds. The EOs composition of Egyptian E. heterophylla ecospecies was comparable to other reported Euphorbia species, although it showed no correlation with Nigerian E. heterophylla ecospecies. The EOs from E. heterophylla aerial parts exhibited significant antioxidant activity. Moreover, a concentration of 100 microL L-1 of the EOs reduced the germination, root, and shoot growth of C. echinatus by about 93.95%, 84.6%, and 57.8%, respectively. Therefore, the EOs from E. heterophylla could be integrated into the control of this weed, as eco-friendly biocontrol method. Further study is needed to characterize their allelopathic activity at field conditions as well as to evaluate their durability and biosafety.
Photodegradation behaviour of multiclass ultraviolet filters in the aquatic environment: Removal strategies and photoproduct identification by liquid chromatography-high resolution mass spectrometry.[Pubmed:30871757]
J Chromatogr A. 2019 Feb 27. pii: S0021-9673(19)30220-1.
Different photodegradation strategies were assessed to remove 21 multiclass organic ultraviolet (UV) filters including benzophenone-, (+)-Camphor-, and p-aminobenzoic acid- derivatives, methoxycinnamates, and salicylates, among others, from the aquatic environment. Direct photolysis under UVA (lambda = 365 nm) and UVC (lambda = 254 nm) radiations and advanced oxidation processes (AOPs) based on heterogeneous UVA/TiO2 photocatalysis and the UVC/H2O2 system were applied for the degradation tests. LC-MS/MS and SPME-GC-MS/MS were employed for the monitoring of the target compound degradations. UVC photolysis provided the highest removal efficiency for most of the studied UV filters with degradation yields higher than 90% after 60 min of light exposure in ultrapure water. This radiation was also applied to different real water matrices (river, sea, and swimming-pool water), showing that the degradation yield was dependent on the water matrix, being more difficult the removal of the target compounds in waters with high organic matter content. In an attempt to accelerate the degradation of the studied compounds in this kind of water matrices, the use of a powerful UVC/H2O2 system improvement the reaction kinetics, showing degradation > 90% for most of the studied UV filters. Besides, up to 19 photoproducts could be identified by HRMS.


