CGS 35066Endothelin-converting enzyme (ECE) inhibitor CAS# 261619-50-5 |
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 261619-50-5 | SDF | Download SDF |
| PubChem ID | 24868306 | Appearance | Powder |
| Formula | C16H16NO6P | M.Wt | 349.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
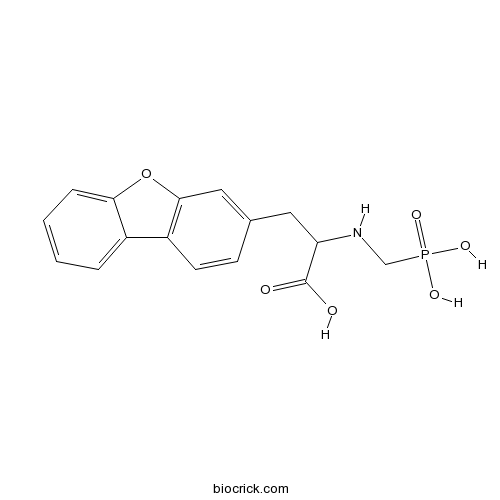
| Chemical Name | 3-dibenzofuran-3-yl-2-(phosphonomethylamino)propanoic acid | ||
| SMILES | C1=CC=C2C(=C1)C3=C(O2)C=C(C=C3)CC(C(=O)O)NCP(=O)(O)O | ||
| Standard InChIKey | CRUVAUSVWLATAE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16NO6P/c18-16(19)13(17-9-24(20,21)22)7-10-5-6-12-11-3-1-2-4-14(11)23-15(12)8-10/h1-6,8,13,17H,7,9H2,(H,18,19)(H2,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent endothelin-converting enzyme (ECE) inhibitor that displays > 100-fold selectivity over neutral endopeptidase 24.11 (IC50 values are 22 and 2300 nM respectively). Blocks the hypertensive effects induced by big ET-1 in vitro and reduces the magnitude of cerebral vasospasm following subarachnoid hemorrhage (SAH). |

CGS 35066 Dilution Calculator

CGS 35066 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.863 mL | 14.3152 mL | 28.6303 mL | 57.2607 mL | 71.5758 mL |
| 5 mM | 0.5726 mL | 2.863 mL | 5.7261 mL | 11.4521 mL | 14.3152 mL |
| 10 mM | 0.2863 mL | 1.4315 mL | 2.863 mL | 5.7261 mL | 7.1576 mL |
| 50 mM | 0.0573 mL | 0.2863 mL | 0.5726 mL | 1.1452 mL | 1.4315 mL |
| 100 mM | 0.0286 mL | 0.1432 mL | 0.2863 mL | 0.5726 mL | 0.7158 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Naproxen Sodium
Catalog No.:BCC6490
CAS No.:26159-34-2
- 3,5-Dihydroxybenzaldehyde
Catalog No.:BCN2257
CAS No.:26153-38-8
- Linderene acetate
Catalog No.:BCN8042
CAS No.:26146-28-1
- Linderene
Catalog No.:BCN2779
CAS No.:26146-27-0
- MB05032
Catalog No.:BCC1731
CAS No.:261365-11-1
- Antiarol rutinoside
Catalog No.:BCN5137
CAS No.:261351-23-9
- Frentizole
Catalog No.:BCC4035
CAS No.:26130-02-9
- 4-O-Feruloylquinic acid
Catalog No.:BCN3352
CAS No.:2613-86-7
- Madurensine
Catalog No.:BCN2092
CAS No.:26126-78-3
- Picrasin B
Catalog No.:BCN5136
CAS No.:26121-56-2
- Rhodojaponin II
Catalog No.:BCN2810
CAS No.:26116-89-2
- Cyanidin-3,5-O-diglucoside chloride
Catalog No.:BCN3116
CAS No.:2611-67-8
- Denudatine
Catalog No.:BCN5406
CAS No.:26166-37-0
- Beesioside Q
Catalog No.:BCC8301
CAS No.:261767-91-3
- 3,19-Dihydroxy-6,23-dioxo-12-ursen-28-oic acid
Catalog No.:BCN1471
CAS No.:261768-88-1
- 6-Methyl-8-prenylnaringenin
Catalog No.:BCN6860
CAS No.:261776-60-7
- 6-Prenylsakuranetin
Catalog No.:BCN7883
CAS No.:261776-61-8
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
Design and synthesis of a potent and selective endothelin-converting enzyme inhibitor, CGS 35066.[Pubmed:11078330]
J Cardiovasc Pharmacol. 2000 Nov;36(5 Suppl 1):S36-9.
CGS 26303 has previously been shown to inhibit human endothelin converting enzyme-1 (ECE-1) with an IC50 of 410 nM and to be efficacious in several animal disease models. However, it is a more potent inhibitor of neutral endopeptidase 24.11 (NEP) with an IC50 of 1 nM. The aim of this study was to optimize CGS 26303 for greater potency and selectivity towards ECE-1 inhibition. The in vivo activity of the compounds was assessed by inhibition of the big endothelin-1 (ET-1)-induced pressor response in anesthetized rats at 90 min after treatment with a dose of 10 mg/kg, i.v. Under these conditions, CGS 26303 inhibited the pressor response to big ET-1 by 50%. Replacement of the biphenyl and tetrazol groups in CGS 26303 with a dibenzofuran and carboxylic acid, respectively, yielded CGS 35066, a potent ECE-1 inhibitor having an IC50 of 22 nM. In contrast, these substitutions markedly weakened the NEP inhibitory activity of the compound to an IC50 of 2.3 microM. CGS 35066 also exhibited a potent and sustained ECE-1 inhibitory activity in vivo, blocking the pressor response to big ET-1 by 84%. Its orally active prodrug, CGS 35339, was obtained by introducing two phenyl groups at the phosphonic acid substituent in CGS 35066. Therefore, CGS 35066 and CGS 35339 represent novel compounds for assessing the pathogenic role of ET-1 overproduction in various disease states.
Effects of benazepril, an angiotensin-converting enzyme inhibitor, combined with CGS 35066, a selective endothelin-converting enzyme inhibitor, on arterial blood pressure in normotensive and spontaneously hypertensive rats.[Pubmed:12193123]
Clin Sci (Lond). 2002 Aug;103 Suppl 48:363S-366S.
Continuous intra-arterial administration of a selective endothelin-converting enzyme (ECE) inhibitor CGS 35066 at a dose of 30 mg/kg decreased the mean arterial blood pressure (MABP) in conscious unrestrained normotensive rats and spontaneously hypertensive rats (SHRs). At that dose, the magnitude of the antihypertensive effects was greater in SHRs than in normotensive rats. Additional administration of an angiotensin-converting enzyme (ACE) inhibitor benazapril (lotensin) further reduced MABP in normotensive rats and completely blocked hypertension in SHRs. However, when the selective ECE inhibitor was subsequently removed, blood pressure was less inhibited in normotenive rats whereas it remained strongly inhibited in SHRs by the ACE inhibitor alone. These results imply that simultaneous treatment with benazepril and CGS 35066 gave additive antihypertensive effects in normotensive rats but not in SHRs, when both compounds were administered at a dose of 30 mg/kg. Our results suggest that: (i) the endothelin (ET) system together with the renin-angiotensin system contribute to the maintenance of blood pressure in normal healthy rats; (ii) while an ECE inhibitor acts as an antihypertensive agent on its own, the sole efficacy of ACE inhibitor at that dose is sufficient to block MABP without the participation of the ET system in SHR.
Prevention and reversal of vasospasm and ultrastructural changes in basilar artery by continuous infusion of CGS 35066 following subarachnoid hemorrhage.[Pubmed:16741051]
Exp Biol Med (Maywood). 2006 Jun;231(6):1069-74.
Endothelin-1, a potent vasoconstrictive peptide, has been implicated in the pathogenesis of cerebral vasospasm following subarachnoid hemorrhage (SAH). The goal of this study was to evaluate the effect of continuous intravenous infusion of a highly selective endothelin-converting enzyme-1 inhibitor, CGS 35066, on the prevention and reversal of cerebral vasospasm following SAH. New Zealand white rabbits were subjected to SAH by injecting autologous arterial blood into the cisterna magna. Infusion of CGS 35066 at dosages of 1, 3, or 10 mg/kg/ day was initiated either 1 hr and 24 hrs later in the prevention and reversal protocols, respectively. Animals were sacrificed by perfusion-fixation 48 hrs after SAH induction. The cross-sectional areas of basilar arteries were measured using computer-assisted videomicroscopy. Ultrastructural changes in basilar arteries were determined using electron microscopy. CGS 35066 significantly prevented and reversed the arterial narrowing after SAH in all three groups. The mean cross-sectional areas of arteries from animals in both the prevention and reversal protocol groups that received 10 mg/kg/day of CGS 35066 did not differ significantly from those of the healthy controls. Histological studies of the basilar artery in the 10 mg/kg/day treatment group did not show pathomorphological changes, such as corrugation of the endothelium seen at 2 days after SAH induction or vacuole formation in the endothelial cells noted in the vehicle-treated SAH group. These findings suggest that CGS 35066 is a promising therapeutic agent for the prevention and reversal of cerebral vasospasm after SAH. It also prevents the pathological changes in vascular walls due to SAH.
Pharmacological properties of CGS 35066, a potent and selective endothelin-converting enzyme inhibitor, in conscious rats.[Pubmed:11078331]
J Cardiovasc Pharmacol. 2000 Nov;36(5 Suppl 1):S40-3.
The purpose of this study was to examine the pharmacologic properties of CGS 35066, a novel aminophosphonate inhibitor of endothelin-converting enzyme-1 (ECE-1). CGS 35066 inhibited the activity of human ECE-1 and rat kidney neutral endopeptidase 24.11 (NEP) in vitro with IC50 values of 22 +/- 0.9 nM and 2.3 +/- 0.03 microM, respectively. The in vivo effects of CGS 35066 were characterized in conscious, catheterized rats. At 30 and 120 min after treatment with vehicle, big endothelin-1 (big ET-1, 0.3 nmol/kg i.v.) produced increases in mean arterial pressure (MAP) of 982 +/- 31 and 992 +/- 43 mmHg x min (area under the curve), respectively. Doses of 0.3, 1.0, 3.0 and 10.0 mg/kg i.v., of CGS 35066 blocked these pressor responses by 61 +/- 7, 78 +/- 4, 93 +/- 4 and 98 +/- 2% at 30 min (p < 0.05 compared with vehicle controls, all doses), and by 29 +/- 7, 63 +/- 5, 63 +/- 5 and 84 +/- 10% at 120 min (p < 0.05, all doses). In contrast, the pressor effect (58 +/- 6 mmHg) of angiotensin-I (300 ng/kg i.v.) was unaffected by the ECE-1 inhibitor (10 mg/kg i.v.) indicating the absence of activity against angiotensin-converting enzyme. In rats infused with atrial natriuretic peptide (ANP), CGS 35066, at 1 mg/kg, had no effect on plasma irANP; however, irANP levels were doubled at a dose of 30 mg/kg. These results demonstrate that CGS 35066 is the most potent and selective ECE inhibitor identified to date.
Potent and selective non-peptidic inhibitors of endothelin-converting enzyme-1 with sustained duration of action.[Pubmed:10669576]
J Med Chem. 2000 Feb 10;43(3):488-504.
Potent and selective non-peptidic inhibitors of human endothelin-converting enzyme-1 (ECE-1) have been designed as potential modulators of endothelin (ET-1) production in vivo. Because of its unique structural characteristics and long duration of action in vivo, the dual ECE-1 and neutral endopeptidase 24.11 (NEP) inhibitor, CGS 26303, was selected as an attractive lead for further optimization of potency and selectivity. Replacement of the P(1)' biphenyl substituent of CGS 26303 by a conformationally restricted 3-dibenzofuranyl group led to more potent and more selective ECE-1 inhibitors, such as the tetrazole 27. The remarkable effect of this P(1)' modification allowed for the first time phosphonomethylcarboxylic acids, such as 29, to display both potent (IC(50) = 22 nM) and selective (104-fold vs NEP) ECE-1 inhibition. Chemoenzymatic syntheses of the new alpha-amino acid (S)-3-dibenzofuran-3-ylalanine intermediate were developed, and improved procedures to generate substituted alpha-aminoalkylphosphonic acids were devised to support the production of various analogues. Although additional gains in intrinsic ECE-1 inhibitory potency could occasionally be achieved by addition of a P(1) side chain, these compounds (e.g. 43a) showed poor functional activity in vivo in the big ET-1 pressor test. Phosphonoalkyl dipeptides featuring 3-dibenzofuranyl groups in both the P(1)' and P(2)' positions were also very potent ECE-1 inhibitors, albeit lacking the desired selectivity against NEP. Functionally, 27and 29 were the two most efficacious compounds from this study, producing sustained inhibition of ECE-1 activity in rats, as measured by their ability to block the hypertensive effects induced by big ET-1. This profile was similar to that of a potent ET(A)/ET(B) dual receptor antagonist, SB 209670. Due to their favorable in vitro and in vivo profiles, 27 (CGS 34043) and 29 (CGS 35066) constitute new pharmacological tools useful in assessing the role of ECE-1 in pathological conditions.


