CALP2Cell-permeable calmodulin antagonist CAS# 261969-04-4 |
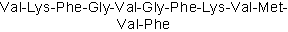
- NPS-2143
Catalog No.:BCC4409
CAS No.:284035-33-2
- NPS-2143 hydrochloride
Catalog No.:BCC1808
CAS No.:324523-20-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 261969-04-4 | SDF | Download SDF |
| PubChem ID | 90471211 | Appearance | Powder |
| Formula | C68H104N14O13S | M.Wt | 1357.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Calcium-like peptide 2 | ||
| Solubility | Soluble to 0.50 mg/ml in 10% ethanol / water | ||
| Sequence | VKFGVGFKVMVF | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-amino-3-methylbutanoyl]amino]hexanoyl]amino]-3-phenylpropanoyl]amino]acetyl]amino]-3-methylbutanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-methylbutanoyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CC(C)C(C(=O)NC(CCCCN)C(=O)NC(CC1=CC=CC=C1)C(=O)NCC(=O)NC(C(C)C)C(=O)NCC(=O)NC(CC2=CC=CC=C2)C(=O)NC(CCCCN)C(=O)NC(C(C)C)C(=O)NC(CCSC)C(=O)NC(C(C)C)C(=O)NC(CC3=CC=CC=C3)C(=O)O)N | ||
| Standard InChIKey | ODWOEJYNFFTQOH-IVGXUUFVSA-N | ||
| Standard InChI | InChI=1S/C68H104N14O13S/c1-40(2)55(71)64(90)76-47(29-19-21-32-69)60(86)78-50(35-44-23-13-10-14-24-44)59(85)72-39-54(84)80-56(41(3)4)65(91)73-38-53(83)74-51(36-45-25-15-11-16-26-45)63(89)75-48(30-20-22-33-70)61(87)81-57(42(5)6)66(92)77-49(31-34-96-9)62(88)82-58(43(7)8)67(93)79-52(68(94)95)37-46-27-17-12-18-28-46/h10-18,23-28,40-43,47-52,55-58H,19-22,29-39,69-71H2,1-9H3,(H,72,85)(H,73,91)(H,74,83)(H,75,89)(H,76,90)(H,77,92)(H,78,86)(H,79,93)(H,80,84)(H,81,87)(H,82,88)(H,94,95)/t47-,48-,49-,50-,51-,52-,55-,56-,57-,58-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable calmodulin (CaM) antagonist that binds to the EF-hand/Ca2+-binding site. CALP2 has been demonstrated to inhibit CaM-dependant phosphodiesterase activity and increase intracellular Ca2+ concentrations by modulating Ca2+-channel activity. CALP2 has also been shown to be a potent activator of alveolar macrophages. |

CALP2 Dilution Calculator

CALP2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- 6-Prenylsakuranetin
Catalog No.:BCN7883
CAS No.:261776-61-8
- 6-Methyl-8-prenylnaringenin
Catalog No.:BCN6860
CAS No.:261776-60-7
- 3,19-Dihydroxy-6,23-dioxo-12-ursen-28-oic acid
Catalog No.:BCN1471
CAS No.:261768-88-1
- Beesioside Q
Catalog No.:BCC8301
CAS No.:261767-91-3
- Denudatine
Catalog No.:BCN5406
CAS No.:26166-37-0
- CGS 35066
Catalog No.:BCC5916
CAS No.:261619-50-5
- Naproxen Sodium
Catalog No.:BCC6490
CAS No.:26159-34-2
- 3,5-Dihydroxybenzaldehyde
Catalog No.:BCN2257
CAS No.:26153-38-8
- Linderene acetate
Catalog No.:BCN8042
CAS No.:26146-28-1
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
- Cl-HOBt
Catalog No.:BCC2829
CAS No.:26198-19-6
- 3alpha-Acetoxy-20-oxo-29-norlupane-23,28-dioic acid
Catalog No.:BCN6507
CAS No.:262272-76-4
- 3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No.:BCN6394
CAS No.:262292-34-2
- H-D-Aib-OH
Catalog No.:BCC3151
CAS No.:2623-91-8
- Debenzoylgalloylpaeoniflorin
Catalog No.:BCC8927
CAS No.:262350-51-6
- Mudanpioside J
Catalog No.:BCC9050
CAS No.:262350-52-7
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- 7,15-Dihydroxypodocarp-8(14)-en-13-one
Catalog No.:BCN1470
CAS No.:262355-96-4
- Cyclofenil
Catalog No.:BCC7839
CAS No.:2624-43-3
- BVD 10
Catalog No.:BCC5882
CAS No.:262418-00-8
- Boc-β-Homo-Pro-OH
Catalog No.:BCC2628
CAS No.:26250-84-0
Calpain-2 Regulates TNF-alpha Expression Associated with Neuropathic Pain Following Motor Nerve Injury.[Pubmed:29477696]
Neuroscience. 2018 Apr 15;376:142-151.
Both calpain-2 (CALP2) and tumor necrosis factor-alpha (TNF-alpha) contribute to persistent bilateral hypersensitivity in animals subjected to L5 ventral root transection (L5-VRT), a model of selective motor fiber injury without sensory nerve damage. However, specific upstream mechanisms regulating TNF-alpha overexpression and possible relationships linking CALP2 and TNF-alpha have not yet been investigated in this model. We examined changes in CALP2 and TNF-alpha protein levels and alterations in bilateral mechanical threshold within 24h following L5-VRT model injury. We observed robust elevation of CALP2 and TNF-alpha in bilateral dorsal root ganglias (DRGs) and bilateral spinal cord neurons. CALP2 and TNF-alpha protein induction by L5-VRT were significantly inhibited by pretreatment using the calpain inhibitor MDL28170. Administration of CALP2 to rats without nerve injury further supported a role of CALP2 in the regulation of TNF-alpha expression. Although clinical trials of calpain inhibition therapy for alleviation of neuropathic pain induced by motor nerve injury have not yet shown success, our observations linking CALP2 and TNF-alpha provide a framework of a systems' approach based perspective for treating neuropathic pain.
Early CALP2 expression and microglial activation are potential inducers of spinal IL-6 up-regulation and bilateral pain following motor nerve injury.[Pubmed:29423951]
J Neurochem. 2018 Apr;145(2):154-169.
Previous work from our laboratory showed that motor nerve injury by lumbar 5 ventral root transection (L5-VRT) led to interleukin-6 (IL-6) over-expression in bilateral spinal cord, and that intrathecal administration of IL-6 neutralizing antibody delayed the induction of mechanical allodynia in bilateral hind paws. However, early events and upstream mechanisms underlying spinal IL-6 expression following L5-VRT require elucidation. The model of L5-VRT was used to induce neuropathic pain, which was assessed with von Frey hairs and the plantar tester in adult male Sprague-Dawley rats. Calpain-2 (CALP2, a calcium-dependent protease) knockdown or over-expression and microglia depletion were conducted intrathecally. Western blots and immunohistochemistry were performed to explore the possible mechanisms. Here, we provide the first evidence that both IL-6 and CALP2 levels are increased in lumbar spinal cord within 30 min following L5-VRT. IL-6 and CALP2 co-localized in both spinal dorsal horn (SDH) and spinal ventral horn. Post-operative (PO) increase in CALP2 in ipsilateral SDH was evident at 10 min PO, preceding increased IL-6 at 20 min PO. Knockdown of spinal CALP2 by intrathecal CALP2-shRNA administration prevented VRT-induced IL-6 overproduction in ipsilateral spinal cord and alleviated bilateral mechanical allodynia. Spinal microglia activation also played a role in early IL-6 up-regulation. Macrophage/microglia markers ED1/Iba1 were increased at 30 min PO, while glial fibrillary acidic protein (astrocyte) and CNPase (oligodendrocyte) markers were not. Increased Iba1 was detected as early as 20 min PO and peaked at 3 days. Morphology changed from a small soma with fine processes in resting cells to an activated ameboid shape. Depletion of microglia using Mac-1-saporin partially prevented IL-6 up-regulation and attenuated VRT-induced bilateral mechanical allodynia. Taken together, our findings provide evidence that increased spinal cord CALP2 and microglia cell activation may have early causative roles in IL-6 over-expression following motor nerve injury. Agents that inhibit CALP2 and/or microglia activation may therefore prove valuable for treating neuropathic pain.
Specific modulation of calmodulin activity induces a dramatic production of superoxide by alveolar macrophages.[Pubmed:14631377]
Lab Invest. 2004 Jan;84(1):29-40.
Airway inflammation is a characteristic feature in airway diseases such as asthma and chronic obstructive pulmonary disease. Oxidative stress, caused by the excessive production of reactive oxygen species by inflammatory cells like macrophages, eosinophils and neutrophils, is thought to be important in the complex pathogenesis of such airway diseases. The calcium-sensing regulatory protein calmodulin (CaM) binds and regulates different target enzymes and proteins, including calcium channels. In the present study, we investigated whether CaM, via the modulation of calcium channel function, influences [Ca(2+)](i) in pulmonary inflammatory cells, and consequently, modulates the production of reactive oxygen species by these cells. This was tested with a peptide termed calcium-like peptide 2 (CALP2), which was previously shown to regulate such channels. Specifically, radical production by purified broncho-alveolar lavage cells from guinea-pigs in response to CALP2 was measured. CALP2 was a strong activator of alveolar macrophages. In contrast, CALP2 was only a mild activator of neutrophils and did not induce radical production by eosinophils. The CALP2-induced radical production was mainly intracellular, and was completely blocked by the NADPH-oxidase inhibitor DPI, the superoxide inhibitor SOD and the CaM antagonist W7. Furthermore, the calcium channel blocker lanthanum partly inhibited the cellular activation by CALP2. We conclude that alveolar macrophages, but not neutrophils or eosinophils, can produce extremely high amounts of reactive oxygen species when stimulated via the calcium/CaM pathway. These results may contribute to new therapeutic strategies against oxidative stress in airway diseases.
Ca2+ sensors modulate asthmatic symptoms in an allergic model for asthma.[Pubmed:12969760]
Eur J Pharmacol. 2003 Aug 22;476(1-2):151-7.
We previously described two novel peptides, Ca2+-like peptide (CALP) 1 and CALP2, which interact with Ca2+-binding EF hand motifs, and therefore have the characteristics to define the role of the Ca2+-sensing regulatory protein calmodulin in asthma. In the present study, the effects of the calcium-like peptides were investigated in an animal model for allergic asthma. For that purpose, sensitized guinea pigs were intratracheally pretreated with CALP1 or CALP2. Thirty minutes later, the animals were challenged with aerosolized ovalbumin. Acute bronchoconstriction was measured as well as characteristic features of asthma 6 and 24 hours (h) after challenge. Neither CALP1 nor CALP2 prevented the anaphylactic response elicited by ovalbumin challenge. However, CALP1 pretreatment attenuated the influx of inflammatory cells in the lungs 6 h after challenge. Furthermore, radical production by these cells was diminished both 6 and 24 h after challenge. Moreover, CALP1 completely inhibited airway hyperresponsiveness in vitro 24 h after challenge. We conclude that CALP1, as a selective calmodulin agonist, inhibits the development of asthmatic features probably via the attenuation of mast cell degranulation and radical production. Specific modulation of calmodulin activity might therefore be a potential new target for the treatment of allergic asthma.
Attenuation of very late antigen-5-mediated adhesion of bone marrow-derived mast cells to fibronectin by peptides with inverted hydropathy to EF-hands.[Pubmed:11145661]
J Immunol. 2001 Jan 15;166(2):861-7.
Release of allergic mediators from mast cells is enhanced by very late Ag (VLA)-5-mediated interaction of these cells with fibronectin. In this report, we show that VLA-5-mediated adhesion of bone marrow-derived mast cells to fibronectin can be induced by two different pathways: first, FcepsilonRI clustering, which depends on calmodulin activation and extracellular Ca(2+), and, second, by Mn(2+) stimulation, which is independent of calmodulin activation and antagonized by Ca(2+). Previous studies have shown the presence of several cation-binding domains in VLA-5 that are homologous to the calcium-binding EF-hands of calmodulin. To show a role for EF-hands of different proteins in VLA-5-mediated adhesion, we used calcium-like peptides (CALP), CALP1 and CALP2, designed to bind to EF-hands based on inverted hydropathy. CALP1 and, more potently, CALP2 inhibited FcepsilonRI-induced adhesion to fibronectin via different mechanisms. The target for the effects of CALP1 and 2 on FcepsilonRI-induced adhesion and degranulation was intracellular and likely involved calmodulin. Interestingly only CALP2 was able to inhibit Mn(2+)-induced calmodulin-independent adhesion by interfering with an extracellular target, which is probably VLA-5. We conclude that CALP1 and 2 can inhibit VLA-5-mediated adhesion of mast cells to fibronectin through binding to EF-hands of multiple proteins, and that these peptides can be used as lead compounds for the development of future therapy against allergy.


