3,5-DihydroxybenzaldehydeCAS# 26153-38-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26153-38-8 | SDF | Download SDF |
| PubChem ID | 94365 | Appearance | Powder |
| Formula | C7H6O3 | M.Wt | 138.12 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5-dihydroxybenzaldehyde | ||
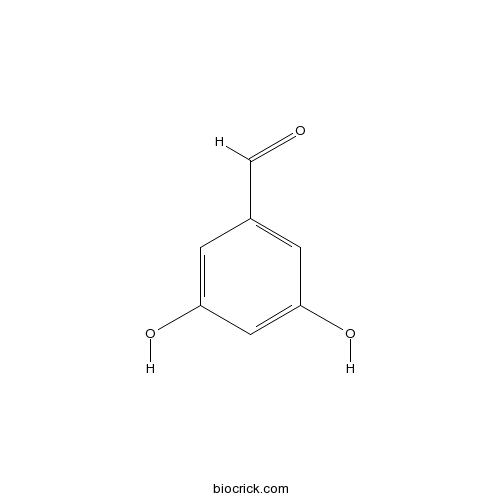
| SMILES | C1=C(C=C(C=C1O)O)C=O | ||
| Standard InChIKey | HAQLHRYUDBKTJG-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Rapid Commun Mass Spectrom, 2010, 24(5):634-642.Liquid chromatographic/electrospray ionization mass spectrometric identification of the oxidation end-products of trans -resveratrol in aqueous solutions.[Reference: WebLink]trans-Resveratrol (3,5,4'-trihydroxystilbene) is a natural polyphenolic compound that exhibits antioxidant properties. Our study aimed at studying the HO*-induced oxidation of resveratrol (100 micromol.L(-1)) in aerated aqueous solutions. Indian Journal of Pharmaceutical Sciences,73,1(2011-11-11), 2011, 73(1):46-56.Stability-indicating HPLC Method for Simultaneous Determination of Terbutaline Sulphate, Bromhexine Hydrochloride and Guaifenesin.[Reference: WebLink]The aim of the present study was the development and subsequent validation of a simple, precise and stability-indicating reversed phase HPLC method for the simultaneous determination of guaifenesin, terbutaline sulphate and bromhexine hydrochloride in the presence of their potential impurities in a single run. |

3,5-Dihydroxybenzaldehyde Dilution Calculator

3,5-Dihydroxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2401 mL | 36.2004 mL | 72.4008 mL | 144.8016 mL | 181.002 mL |
| 5 mM | 1.448 mL | 7.2401 mL | 14.4802 mL | 28.9603 mL | 36.2004 mL |
| 10 mM | 0.724 mL | 3.62 mL | 7.2401 mL | 14.4802 mL | 18.1002 mL |
| 50 mM | 0.1448 mL | 0.724 mL | 1.448 mL | 2.896 mL | 3.62 mL |
| 100 mM | 0.0724 mL | 0.362 mL | 0.724 mL | 1.448 mL | 1.81 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Linderene acetate
Catalog No.:BCN8042
CAS No.:26146-28-1
- Linderene
Catalog No.:BCN2779
CAS No.:26146-27-0
- MB05032
Catalog No.:BCC1731
CAS No.:261365-11-1
- Antiarol rutinoside
Catalog No.:BCN5137
CAS No.:261351-23-9
- Frentizole
Catalog No.:BCC4035
CAS No.:26130-02-9
- 4-O-Feruloylquinic acid
Catalog No.:BCN3352
CAS No.:2613-86-7
- Madurensine
Catalog No.:BCN2092
CAS No.:26126-78-3
- Picrasin B
Catalog No.:BCN5136
CAS No.:26121-56-2
- Rhodojaponin II
Catalog No.:BCN2810
CAS No.:26116-89-2
- Cyanidin-3,5-O-diglucoside chloride
Catalog No.:BCN3116
CAS No.:2611-67-8
- Chicago Sky Blue 6B
Catalog No.:BCC6816
CAS No.:2610-05-1
- Reneilmol
Catalog No.:BCN5135
CAS No.:260968-11-4
- Naproxen Sodium
Catalog No.:BCC6490
CAS No.:26159-34-2
- CGS 35066
Catalog No.:BCC5916
CAS No.:261619-50-5
- Denudatine
Catalog No.:BCN5406
CAS No.:26166-37-0
- Beesioside Q
Catalog No.:BCC8301
CAS No.:261767-91-3
- 3,19-Dihydroxy-6,23-dioxo-12-ursen-28-oic acid
Catalog No.:BCN1471
CAS No.:261768-88-1
- 6-Methyl-8-prenylnaringenin
Catalog No.:BCN6860
CAS No.:261776-60-7
- 6-Prenylsakuranetin
Catalog No.:BCN7883
CAS No.:261776-61-8
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
- Isotaxiresinol
Catalog No.:BCN4660
CAS No.:26194-57-0
- CALP2
Catalog No.:BCC5898
CAS No.:261969-04-4
- CALP3
Catalog No.:BCC5900
CAS No.:261969-05-5
Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes.[Pubmed:22795593]
Ecotoxicol Environ Saf. 2012 Sep;83:71-8.
Exposure of the skin to ultraviolet B (UVB) radiation leads to epidermal damage and the generation of reactive oxygen species (ROS) in skin cells, including keratinocytes. Therefore, the photo-protective effect of 3-bromo-4, 5-dihydroxybenzaldehyde (BDB) against UVB was assessed in human HaCaT keratinocytes exposed to UVB radiation in vitro. BDB restored cell viability, which decreased upon exposure to UVB radiation. BDB exhibited scavenging activity against 1, 1-diphenyl-2-picrylhydrazyl radicals, intracellular ROS induced by hydrogen peroxide (H(2)O(2)) or UVB radiation, the superoxide anion generated by the xanthine/xanthine oxidase system, and the hydroxyl radical generated by the Fenton reaction (FeSO(4)+H(2)O(2)). Moreover, BDB absorbed UVB and decreased injury resulting from UVB-induced oxidative stress to lipids, proteins and DNA. Finally, BDB reduced UVB-induced apoptosis, as exemplified by fewer apoptotic bodies and a reduction in DNA fragmentation. Taken together, these results suggest that BDB protects human keratinocytes against UVB-induced oxidative stress by scavenging ROS and absorbing UVB rays, thereby reducing injury to cellular components.


