BenzalacetoneCAS# 122-57-6 |
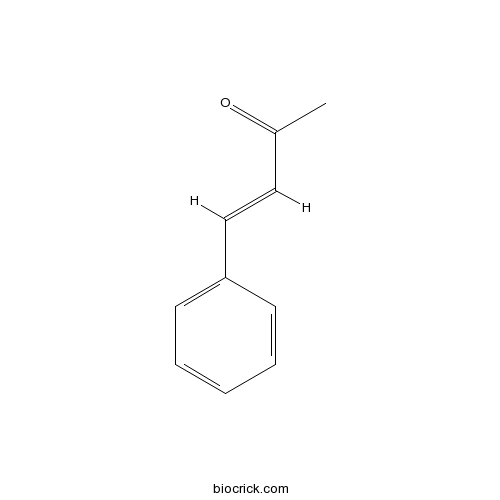
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122-57-6 | SDF | Download SDF |
| PubChem ID | 637759 | Appearance | Powder |
| Formula | C10H10O | M.Wt | 146.19 |
| Type of Compound | Impurities | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-4-phenylbut-3-en-2-one | ||
| SMILES | CC(=O)C=CC1=CC=CC=C1 | ||
| Standard InChIKey | BWHOZHOGCMHOBV-BQYQJAHWSA-N | ||
| Standard InChI | InChI=1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzalacetone Dilution Calculator

Benzalacetone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.8404 mL | 34.2021 mL | 68.4041 mL | 136.8083 mL | 171.0103 mL |
| 5 mM | 1.3681 mL | 6.8404 mL | 13.6808 mL | 27.3617 mL | 34.2021 mL |
| 10 mM | 0.684 mL | 3.4202 mL | 6.8404 mL | 13.6808 mL | 17.101 mL |
| 50 mM | 0.1368 mL | 0.684 mL | 1.3681 mL | 2.7362 mL | 3.4202 mL |
| 100 mM | 0.0684 mL | 0.342 mL | 0.684 mL | 1.3681 mL | 1.7101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,2,3,4-Tetrahydro-1-acetylquinoline
Catalog No.:BCN9648
CAS No.:4169-19-1
- tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate
Catalog No.:BCN9647
CAS No.:289042-12-2
- 5-[2-[[(4-Methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-3-(phenylmethoxy)-2-[(phenylmethoxy)methyl]cyclopentanol
Catalog No.:BCN9646
CAS No.:142217-78-5
- Teriflunomide
Catalog No.:BCN9645
CAS No.:163451-81-8
- 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
Catalog No.:BCN9644
CAS No.:184177-83-1
- Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester
Catalog No.:BCN9643
CAS No.:149809-43-8
- 3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester
Catalog No.:BCN9642
CAS No.:211915-84-3
- 6-Des(1-methyl-2-benzimidazolyl)-6-carboxy telmisartan
Catalog No.:BCN9641
CAS No.:884330-12-5
- 2-Iodo-3-oxo-4-azaandrostane-17-carboxylic acid
Catalog No.:BCN9640
CAS No.:104239-97-6
- Diethyl 2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate 4-methylbenzenesulfonate
Catalog No.:BCN9639
CAS No.:165049-28-5
- Norgestrel
Catalog No.:BCN9638
CAS No.:6533-00-2
- Methyl 1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetra-hydro-1H-pyrido[3,4-b]indole-3-carboxylate
Catalog No.:BCN9637
CAS No.:171489-59-1
- Bisdemethoxycucurmin
Catalog No.:BCN9650
CAS No.:24939-16-0
- N,N'-Bis(2-hydroxyethyl)ethylenediamine
Catalog No.:BCN9651
CAS No.:4439-20-7
- 1-Benzyl D-aspartate
Catalog No.:BCN9652
CAS No.:79337-40-9
- 17-Ethylendioxyandrosta-1,4-dien-3-one
Catalog No.:BCN9653
CAS No.:2398-63-2
- Acid Orange 7
Catalog No.:BCN9654
CAS No.:633-96-5
- Saccharin sodium
Catalog No.:BCN9655
CAS No.:128-44-9
- Orange IV
Catalog No.:BCN9656
CAS No.:554-73-4
- Ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCN9657
CAS No.:429659-01-8
- Dexamethasone 21-phosphate disodium salt
Catalog No.:BCN9658
CAS No.:2392-39-4
- benzyl hydrogen (3-(((2R,3S)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-3-(4-fluorophenyl)morpholino)methyl)-5-imino-4,5-dihydro-1H-1,2,4-triazol-1-yl)phosphonate
Catalog No.:BCN9659
CAS No.:889852-02-2
- Plucheoside B aglycone
Catalog No.:BCN9660
CAS No.:393862-19-6
- Trolline
Catalog No.:BCN9661
CAS No.:1021950-79-7
Synthesis of the character impact compound raspberry ketone and additional flavoring phenylbutanoids of biotechnological interest with Corynebacterium glutamicum.[Pubmed:32316987]
Microb Cell Fact. 2020 Apr 21;19(1):92.
BACKGROUND: The phenylbutanoid 4-(4-hydroxyphenyl)butan-2-one, commonly known as raspberry ketone, is responsible for the typical scent and flavor of ripe raspberries. Chemical production of nature-identical raspberry ketone is well established as this compound is frequently used to flavor food, beverages and perfumes. However, high demand for natural raspberry ketone, but low natural abundance in raspberries, render raspberry ketone one of the most expensive natural flavoring components. RESULTS: In this study, Corynebacterium glutamicum was engineered for the microbial synthesis of the character impact compound raspberry ketone from supplemented p-coumaric acid. In this context, the NADPH-dependent curcumin/dihydrocurcumin reductase CurA from Escherichia coli was employed to catalyze the final step of raspberry ketone synthesis as it provides a hitherto unknown Benzalacetone reductase activity. In combination with a 4-coumarate: CoA ligase from parsley (Petroselinum crispum) and a monofunctional Benzalacetone synthase from Chinese rhubarb (Rheum palmatum), CurA constitutes the synthetic pathway for raspberry ketone synthesis in C. glutamicum. The resulting strain accumulated up to 99.8 mg/L (0.61 mM) raspberry ketone. In addition, supplementation of other phenylpropanoids allowed for the synthesis of two other naturally-occurring and flavoring phenylbutanoids, zingerone (70 mg/L, 0.36 mM) and benzylacetone (10.5 mg/L, 0.07 mM). CONCLUSION: The aromatic product portfolio of C. glutamicum was extended towards the synthesis of the flavoring phenylbutanoids raspberry ketone, zingerone and benzylacetone. Key to success was the identification of CurA from E. coli having a Benzalacetone reductase activity. We believe, that the constructed C. glutamicum strain represents a versatile platform for the production of natural flavoring phenylbutanoids at larger scale.
Construction of synthetic pathways for raspberry ketone production in engineered Escherichia coli.[Pubmed:30915501]
Appl Microbiol Biotechnol. 2019 May;103(9):3715-3725.
Raspberry ketone is an important ingredient in the flavor and fragrance industries. Due to its low content in fruits and vegetables, the production of natural raspberry ketone using heterologous synthesis in microbial strains is recently attracting increased attention. In this work, a heterologous pathway to produce raspberry ketone from p-coumaric acid, including 4-coumarate: CoA ligase (4CL), Benzalacetone synthase (BAS), and raspberry ketone/zingerone synthase (RZS1) from plants, was successfully assembled in Escherichia coli. When the RZS1 gene was introduced into E. coli and co-expressed with two other genes, the intermediate 4-hydroxybenzylidene acetone in the pathway was almost completely transformed into a raspberry ketone. Substituting TB medium for M9 medium increased raspberry ketone titers by 3-4 times. Furthermore, the heterologous pathway was partitioned into two modules; module one produced p-coumaroyl-CoA from p-coumaric acid by 4CL, and module two produced raspberry ketone from coumaroyl-CoA by the action of BAS and RZS1. Optimizing the balanced expression of the two modules, it was shown that moderate expression of module one and high expression of module two was the best combination to enhance raspberry ketone production. The engineered strain CZ-8 reached 90.97 mg/l of raspberry ketone, which was 12 times higher than previously reported. In addition, the preferred approach of the heterologous pathway was related to the heterologous genes from different sources; for example, 4CL from Arabidopsis thaliana seemed to be more suitable for raspberry ketone production than that from Petroselinum crispum. This work paves an alternative way for future economic production of natural raspberry ketone.
In silico approaches illustrate the evolutionary pattern and protein-small molecule interactions of quinolone synthase from Aegle marmelos Correa.[Pubmed:29308712]
J Biomol Struct Dyn. 2019 Jan;37(1):195-209.
Quinolone synthase from Aegle marmelos (AmQNS) is a Rutacean-specific plant type III polyketide synthase that synthesizes quinolone, acridone, and Benzalacetone with therapeutic potential. Simple architecture and broad substrate affinity of AmQNS make it as one of the target enzymes to produce novel structural scaffolds. Another unique feature of AmQNS despite its high similarity to acridone forming type III polyketide synthase from Citrus microcarpa is the variation in the product formation. Hence, to explore the characteristic features of AmQNS, an in-depth sequence and structure-based bioinformatics analyses were performed. Our studies indicated that AmQNS and its nearest homologs have evolved by a series of gene duplication events and strong purifying selection pressure constrains them in the evolutionary process. Additionally, some amino acid alterations were identified in the functionally important region(s), which can contribute to the functional divergence of the enzyme. Prediction of favorable amino acid substitutions will be advantageous in the metabolic engineering of AmQNS for the production of novel compounds. Furthermore, comparative modeling and docking studies were utilized to investigate the structural behavior and small molecule interaction pattern of AmQNS. The observations and results reported here are crucial for advancing our understanding of AmQNS's phylogenetic position, selection pressure, evolvability, interaction pattern and thus providing the foundation for further studies on the structural and reaction mechanism.
3, 4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways.[Pubmed:28644965]
Int Immunopharmacol. 2017 Sep;50:77-86.
3, 4-DihydroxyBenzalacetone (DBL) is a constituent of Phellinus linteus. This study demonstrated the protective effect of DBL on lipopolysaccharide (LPS)-induced acute lung injuries in mice. Pretreatment with DBL significantly improved LPS-induced histological alterations in lung tissues. In addition, DBL markedly reduced the total cell number, the leukocytes, the protein concentrations, and decreased the release of nitrite, tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6 and the activities of matrix metalloproteinase (MMP)-2 and -9 in the bronchoalveolar lavage fluid. DBL also inhibited the W/D ratio and myeloperoxidase activity in the lung tissues. Western blot analysis indicated DBL efficiently blocked the protein expressions of inducible nitric oxide synthase, cyclooxygenase-2, MMP-2, MMP-9, and the phosphorylation of mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K), AKT, Toll-like receptor 4 (TLR4) and nuclear factor (NF)-kappaB. Moreover, DBL enhanced the expression of anti-oxidant proteins, such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx). Based on our results, DBL might be a potential target for attenuating tissue oxidative injuries and nonspecific pulmonary inflammation.
A One-pot Condensation for Synthesis 2-methyl-4-phenylpyrano[3, 2-c] chromen-5(4H)-one and Synthesis of Warfarin by Ionic Liquid Catalysis.[Pubmed:27980569]
Iran J Pharm Res. 2016 Summer;15(3):343-367.
The anticoagulant racemic warfarin is synthesized by the Michael addition of 4-hydroxycoumarin with Benzalacetone in the present of equimolar amounts of imidazolium based ionic liquids [bmim] BF4 and [bmim] Br and other reaction solvents such as H2O, pyridine and ammonia in five different tests. Also synthesis of a derivative of warfarin (2-methyl-4-phenyl pyrano [3, 2-c] chromen-5(4H)-one) under solvent-free condition is reported. In this paper, we show the potential that ionic liquid have for the development of green methods for the formation of the C-C bond by reaction condensations without catalysts and organic solvents. A ()green method(,) according to the well-known principles, must reduce or eliminate the use or generation of unsafe substances. The work-up procedures were fairly simple and the products dont require further purification.
A mechanistic insight into the effect of piperidine as an organocatalyst on the [3 + 2] cycloaddition reaction of benzalacetone with phenyl azide from a computational study.[Pubmed:27403925]
Org Biomol Chem. 2016 Jul 26;14(30):7324-33.
Several transition structures (TSs) for catalyst-free [3 + 2] cycloaddition and two plausible mechanistic pathways for the organocatalyzed [3 + 2] cycloaddition (32CA) between Benzalacetone and phenyl azide were located by quantum chemistry methods. Calculations were carried out with B3LYP, MPWB1K and M06-2X functionals using 6-31G(d) and 6-311G(d,p) basis sets in gas and solvent phases. The calculated activation barriers imply that the lowest barrier pathway is the catalyzed process producing 3-regioisomers through the iminium intermediate and not through the dienamine route. Electronic displacements along the reaction path have been examined using a topological analysis of the electron-localization function (ELF). ELF topological analyses along the intrinsic reaction coordinates (IRC) of both catalyzed and uncatalyzed 32CA reactions indicated that while the first C1-N1 single bond is formed as a dative bond, the formation of the second C2-N3 bond takes place via a C-to-N coupling between the interacting centers of the reagents. Moreover, the ELF analyses imply that the reaction mechanism is a two-stage one-step process in the presence of a piperidine organocatalyst, while bond formation in an uncatalyzed process is almost synchronous.
Synthesis of Unnatural 2-Substituted Quinolones and 1,3-Diketones by a Member of Type III Polyketide Synthases from Huperzia serrata.[Pubmed:27399835]
Org Lett. 2016 Aug 5;18(15):3550-3.
A curcuminoids, Benzalacetone-, and quinolone-producing type III polyketide synthase (HsPKS3) from Huperzia serrata uniquely catalyzes the formation of unnatural 2-substituted quinolones and 1,3-diketones via head-to-head condensation of two completely different substrates. The broad range of substrate tolerance of HsPKS3 facilitates accessing structurally diverse 2-substituted quinolones and 1,3-diketones.


