NorgestrelCAS# 6533-00-2 |
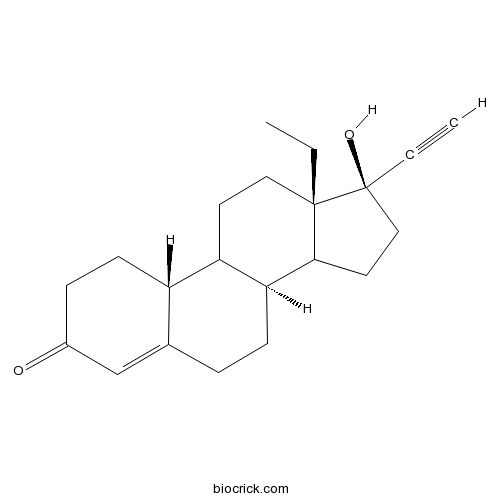
- L-Norgestrel
Catalog No.:BCC9105
CAS No.:797-64-8
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6533-00-2 | SDF | Download SDF |
| PubChem ID | 126968545 | Appearance | Powder |
| Formula | C21H28O2 | M.Wt | 312.4 |
| Type of Compound | Impurities | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8S,10R,13S,17R)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CCC12CCC3C(C1CCC2(C#C)O)CCC4=CC(=O)CCC34 | ||
| Standard InChIKey | WWYNJERNGUHSAO-YHKGGNDHSA-N | ||
| Standard InChI | InChI=1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17?,18-,19?,20-,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Norgestrel Dilution Calculator

Norgestrel Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl 1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetra-hydro-1H-pyrido[3,4-b]indole-3-carboxylate
Catalog No.:BCN9637
CAS No.:171489-59-1
- cis-1,2,3,4-Tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride
Catalog No.:BCN9636
CAS No.:171752-68-4
- Telmisartan impurity G
Catalog No.:BCN9635
CAS No.:144702-27-2
- Telmisartan amide
Catalog No.:BCN9634
CAS No.:915124-86-6
- 2-(2-Aminopropan-2-yl)-N-(4-fluorobenzyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidine-4-carboxamide
Catalog No.:BCN9633
CAS No.:518048-03-8
- Benzyl(2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)carbamate
Catalog No.:BCN9632
CAS No.:518048-02-7
- 1-(2-Chloroacetyl)pyrrolidine-2-carbonitrile
Catalog No.:BCN9631
CAS No.:207557-30-5
- Tricin 7-O-glucoside
Catalog No.:BCN9630
CAS No.:32769-01-0
- Pedaliin
Catalog No.:BCN9629
CAS No.:22860-72-6
- Lutonarin
Catalog No.:BCN9628
CAS No.:35450-86-3
- (S)-4-Methoxydalbergione
Catalog No.:BCN9627
CAS No.:2543-95-5
- Neochlorogenin
Catalog No.:BCN9626
CAS No.:511-91-1
- Diethyl 2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate 4-methylbenzenesulfonate
Catalog No.:BCN9639
CAS No.:165049-28-5
- 2-Iodo-3-oxo-4-azaandrostane-17-carboxylic acid
Catalog No.:BCN9640
CAS No.:104239-97-6
- 6-Des(1-methyl-2-benzimidazolyl)-6-carboxy telmisartan
Catalog No.:BCN9641
CAS No.:884330-12-5
- 3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester
Catalog No.:BCN9642
CAS No.:211915-84-3
- Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester
Catalog No.:BCN9643
CAS No.:149809-43-8
- 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
Catalog No.:BCN9644
CAS No.:184177-83-1
- Teriflunomide
Catalog No.:BCN9645
CAS No.:163451-81-8
- 5-[2-[[(4-Methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-3-(phenylmethoxy)-2-[(phenylmethoxy)methyl]cyclopentanol
Catalog No.:BCN9646
CAS No.:142217-78-5
- tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate
Catalog No.:BCN9647
CAS No.:289042-12-2
- 1,2,3,4-Tetrahydro-1-acetylquinoline
Catalog No.:BCN9648
CAS No.:4169-19-1
- Benzalacetone
Catalog No.:BCN9649
CAS No.:122-57-6
- Bisdemethoxycucurmin
Catalog No.:BCN9650
CAS No.:24939-16-0
Long-term efficacy and feasibility of levonorgestrel-releasing intrauterine device use in patients with adenomyosis.[Pubmed:32481439]
Medicine (Baltimore). 2020 May 29;99(22):e20421.
To evaluate the efficacy and feasibility of levoNorgestrel-releasing intrauterine device (LNG-IUD) use longer than 5 years in women with adenomyosis.Data were retrospectively collected from patients who were treated with LNG-IUD longer than 5 years at the Chungnam National University hospital for adenomyosis diagnosed with ultrasonography from January 2006 to November 2013.A total of 131 patients who were diagnosed with adenomyosis had treated with LNG-IUD longer than 5 years. The mean duration of keeping 1 device without replacement was 58.35 +/- 15.98 months, and total duration of LNG-IUD treatment was 83.86 +/- 23.88 months. A total of 51 patients stopped using LNG-IUD after 5 years and the mean age at the time of LNG-IUD removal was 52.46 +/- 6.9. LNG-IUD treatment had a significant effect on both menorrhagia and dysmenorrhea starting from the first month of insertion (P < .01), which persisted until 6 years when the effect started to plateau. The decrease in uterine volume was not consistent during the treatment period. The uterine volume decreased significantly only in the first and second year of LNG-IUD treatment and then from eighth to tenth year of LNG-IUD treatment (P < .05). Adverse events after insertion of LNG-IUD decreased significantly after 5 years.LNG-IUD treatment longer than 5 years is an effective and feasible method for patients diagnosed with adenomyosis.
Deep eutectic solvent-based magnetic colloidal gel assisted magnetic solid-phase extraction: A simple and rapid method for the determination of sex hormones in cosmetic skin care toners.[Pubmed:32417516]
Chemosphere. 2020 Sep;255:127004.
A simple rapid and efficient deep eutectic solvent-based magnetic colloidal gel (DES-MCG) assisted magnetic solid-phase extraction (MSPE) method followed by high performance liquid chromatography with a diode array detector (HPLC-DAD) was established for determination of four sex hormones (including ethinylestradiol, Norgestrel, megestrol acetate and medroxyprogesterone acetate) in cosmetic skin care toners. The DES-MCG with the desirable advantages of high adsorbing ability was prepared by combining choline chloride/urea deep eutectic solvent and magnetic multiwalled carbon nanotubes (MMWCNTs). The synthesized DES-MCG was characterized using fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and vibrating sample magnetometry (VSM). The cosmetic skin care toners were concentrated by a rotary evaporator and the obtained solutions were further purified by DES-MCG assisted magnetic solid-phase extraction. Response surface methodology (RSM) was applied for efficient optimization of the main variables in the extraction procedure. Under the optimized conditions, method detection limits and method quantitation limits were in the range of 1.2-6.6 ng mL(-1) and 4.4-26.6 ng mL(-1), respectively. The recoveries of the four sex hormones in different cosmetic skin care toners ranged from 80.1% to 118.8% and the precisions were no more than 0.35%. The developed method was successfully applied for the determination of sex hormones in cosmetic skin care toners.
[Effects of LNG-IUS on sexual function and sexual quality in women of childbearing age].[Pubmed:32344499]
Zhonghua Yi Xue Za Zhi. 2020 Apr 28;100(16):1255-1259.
Objective: To investigate the effects of levoNorgestrel releasing intrauterine system (LNG-IUS) on sexual function and sexual quality in women of childbearing age. Methods: A total of 203 healthy women who were using IUD for long-term contraception were enrolled in the study. Among them, 130 were placed LNG-IUS as the study group and 73 were placed the copper intrauterine devices (Cu-IUDs) as the control group. The two groups were further divided into three subgroups by age. The basic information and questionnaires were adopted before and 2 years after using IUDs, including age, the time of using IUD, side-effects after using IUD, frequency and satisfaction of sex after using IUD. The Female Sexual Function Index (FSFI) was evaluated on 2 years after. Results: In the 30-39 age subgroup, the frequency of sex was significantly decreased after using LNG-IUS (P<0.05). Rests of the subgroup shows no significantly different in the frequency of sex (P>0.05). The sexual satisfaction in all subgroups also shows no significantly different before and after using IUDs (P>0.05). There was no significant difference in the individual score and total scores of FSFI between the study group and control group (P>0.05). Conclusion: The LNG-IUS has no adverse effects on female sexual function and sexual quality in the reproductive age.
Sorption and desorption of seven steroidal synthetic progestins in five agricultural soil-water systems.[Pubmed:32272348]
Ecotoxicol Environ Saf. 2020 Jun 15;196:110586.
Manure fertilization and wastewater irrigation can introduce the biologically potent synthetic progestins into agricultural soils, causing endocrine disruption in organisms of nearby surface waters. Therefore, this study investigated the sorption and desorption potential of etonogestrel, medroxyprogesterone, gestodene, Norgestrel, cyproterone acetate, levoNorgestrel, and dienogest in five agricultural soil-water systems. Sorption data were well-described by the linear sorption model. In most batch systems, cyproterone acetate exhibited the highest affinities for soils, followed by etonogestrel, medroxyprogesterone, levoNorgestrel, gestodene, Norgestrel, and dienogest. The sorption magnitudes (logKoc or logKd) were significantly correlated with the progestin hydrophobicities (R(2) = 0.72-0.86, p < 0.05). The Kd values of the progestins were also significantly correlated with organic carbon content and pore volumes of the soils (R(2) = 0.68-0.98, p < 0.05). In addition, 0.5 M urea resulted in 3-19% decreases in Kd values of the progestins. Taken together, these data indicated that hydrophobic partitioning interaction, hydrogen bonding interaction, and pore filling were the sorption mechanisms for the progestins in soil-water systems. No significant desorption hysteresis was observed for the progestins, indicating that they can be readily desorbed under rainfall or irrigation events. Based on the sorption and desorption data, we estimated the dynamic transport of the progestins in conventional agricultural management systems, and predicted the concentrations of the progestins as a function of soil-sorbed concentration, water-soil ratio, and dilution factor of receiving waters. This study will improve the understanding of the risks posed by the progestins under field-scale hydrological conditions.
Relative Risk of Cervical Neoplasms Among Copper and Levonorgestrel-Releasing Intrauterine System Users.[Pubmed:31923062]
Obstet Gynecol. 2020 Feb;135(2):319-327.
OBJECTIVE: To evaluate the relative risk of cervical neoplasms among copper intrauterine device (Cu IUD) and levoNorgestrel-releasing intrauterine system (LNG-IUS) users. METHODS: We performed a retrospective cohort analysis of 10,674 patients who received IUDs at Columbia University Medical Center. Our data were transformed to a common data model and are part of the Observational Health Data Sciences and Informatics network. The cohort patients and outcomes were identified by a combination of procedure codes, condition codes, and medication exposures in billing and claims data. We adjusted for confounding with propensity score stratification and propensity score 1:1 matching. RESULTS: Before propensity score adjustment, the Cu IUD cohort included 8,274 patients and the LNG-IUS cohort included 2,400 patients. The median age for both cohorts was 29 years at IUD placement. More than 95% of the LNG-IUS cohort used a device with 52 mg LNG. Before propensity score adjustment, we identified 114 cervical neoplasm outcomes. Seventy-seven (0.9%) cervical neoplasms were in the Cu IUD cohort and 37 (1.5%) were in the LNG-IUS cohort. The propensity score matching analysis identified 7,114 Cu IUD and 2,174 LNG-IUS users, with covariate balance achieved over 16,827 covariates. The diagnosis of high-grade cervical neoplasia was 0.7% in the Cu IUD cohort and 1.8% in the LNG-IUS cohort (2.4 [95% CI 1.5-4.0] cases/1,000 person-years and 5.2 [95% CI 3.7-7.1] cases/1,000 person-years, respectively). The relative risk of high-grade cervical neoplasms among Cu IUD users was 0.38 (95% CI 0.16-0.78, P<.02) compared with LNG-IUS users. By inspection, the Kaplan-Meier curves for each cohort diverged over time. CONCLUSION: Copper IUD users have a lower risk of high-grade cervical neoplasms compared with LNG-IUS users. The relative risk of cervical neoplasms of LNG-IUS users compared with the general population is unknown.


