TrollineCAS# 1021950-79-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1021950-79-7 | SDF | Download SDF |
| PubChem ID | 21574476 | Appearance | Powder |
| Formula | C12H13NO3 | M.Wt | 219.24 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
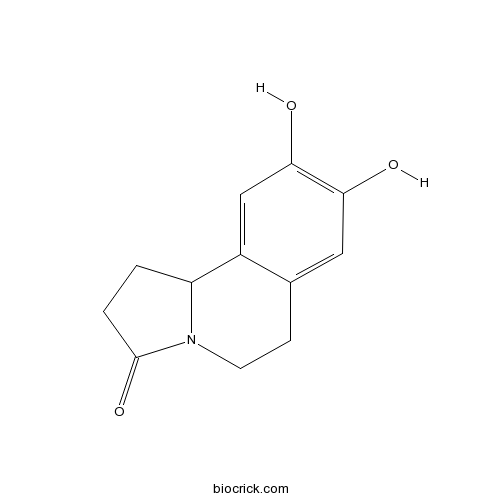
| Chemical Name | 8,9-dihydroxy-2,5,6,10b-tetrahydro-1H-pyrrolo[2,1-a]isoquinolin-3-one | ||
| SMILES | C1CC(=O)N2C1C3=CC(=C(C=C3CC2)O)O | ||
| Standard InChIKey | LJIDRFNRDLYHNC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H13NO3/c14-10-5-7-3-4-13-9(1-2-12(13)16)8(7)6-11(10)15/h5-6,9,14-15H,1-4H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Trolline Dilution Calculator

Trolline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5612 mL | 22.8061 mL | 45.6121 mL | 91.2242 mL | 114.0303 mL |
| 5 mM | 0.9122 mL | 4.5612 mL | 9.1224 mL | 18.2448 mL | 22.8061 mL |
| 10 mM | 0.4561 mL | 2.2806 mL | 4.5612 mL | 9.1224 mL | 11.403 mL |
| 50 mM | 0.0912 mL | 0.4561 mL | 0.9122 mL | 1.8245 mL | 2.2806 mL |
| 100 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Plucheoside B aglycone
Catalog No.:BCN9660
CAS No.:393862-19-6
- benzyl hydrogen (3-(((2R,3S)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-3-(4-fluorophenyl)morpholino)methyl)-5-imino-4,5-dihydro-1H-1,2,4-triazol-1-yl)phosphonate
Catalog No.:BCN9659
CAS No.:889852-02-2
- Dexamethasone 21-phosphate disodium salt
Catalog No.:BCN9658
CAS No.:2392-39-4
- Ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCN9657
CAS No.:429659-01-8
- Orange IV
Catalog No.:BCN9656
CAS No.:554-73-4
- Saccharin sodium
Catalog No.:BCN9655
CAS No.:128-44-9
- Acid Orange 7
Catalog No.:BCN9654
CAS No.:633-96-5
- 17-Ethylendioxyandrosta-1,4-dien-3-one
Catalog No.:BCN9653
CAS No.:2398-63-2
- 1-Benzyl D-aspartate
Catalog No.:BCN9652
CAS No.:79337-40-9
- N,N'-Bis(2-hydroxyethyl)ethylenediamine
Catalog No.:BCN9651
CAS No.:4439-20-7
- Bisdemethoxycucurmin
Catalog No.:BCN9650
CAS No.:24939-16-0
- Benzalacetone
Catalog No.:BCN9649
CAS No.:122-57-6
- (±)-Lariciresinol
Catalog No.:BCN9662
CAS No.:105367-81-5
- (±)-Magnolin
Catalog No.:BCN9663
CAS No.:1275595-33-9
- (+)-Hannokinol
Catalog No.:BCN9664
CAS No.:408324-76-5
- Polyhydroxybutyrate
Catalog No.:BCN9665
CAS No.:26744-04-7
- Phomaligol A
Catalog No.:BCN9666
CAS No.:152204-32-5
- Euglobal Ia2
Catalog No.:BCN9667
CAS No.:77794-63-9
- 4-Chloropinselin
Catalog No.:BCN9668
CAS No.:104022-83-5
- Herbaridine B
Catalog No.:BCN9669
CAS No.:1151511-05-5
- 18-Hydroxytritriacontan-16-one
Catalog No.:BCN9670
CAS No.:97191-42-9
- (2Z,6Z,10E,14E,18E)-Farnesylfarnesol
Catalog No.:BCN9671
CAS No.:90695-03-7
- 5-O-Ethylcleroindicin D
Catalog No.:BCN9672
CAS No.:488138-32-5
- 3-Hydroxychimaphilin
Catalog No.:BCN9673
CAS No.:33253-99-5
Anti-influenza A virus mechanism of three representative compounds from Flos Trollii via TLRs signaling pathways.[Pubmed:32004628]
J Ethnopharmacol. 2020 May 10;253:112634.
ETHNOPHARMACOLOGICAL RELEVANCE: Flos Trollii is the dried flowers of Trollius chinensis. It has been used as a traditional herbal medicine for the treatment of upper respiratory tract infection, tonsillitis and pharyngitis in China for a long history. Veratric acid, vitexin, and Trolline are the representative compounds of phenolic acids, flavonoids and alkaloids in this herbal medicine. All of these three compounds show antiviral activity which is related to the efficacy of Flos Trollii. AIM OF THE STUDY: To investigate the anti-influenza A virus mechanism of the three representative compounds from the perspective of regulating TLRs signaling pathways, so as to understand the relevant efficacy of Flos Trollii. MATERIALS AND METHODS: Influenza A virus A/FM/1/47 (H1N1) and mouse peritoneal macrophages (RAW264.7) were used in the whole process of investigation. MTT assay was conducted to select the appropriate experimental concentrations of the three compounds on RAW264.7 cells. Western blot, RT-PCR, and ELISA assays were performed to determine the protein and mRNA expression of key factors and related inflammatory factors of TLRs signaling pathways. Griess method was employed to detect the production of NO. RESULTS: The three representative compounds reduced the inflammatory factors including NO, IL-6, and TNF-alpha and enhanced the production of IFN-beta through dynamically regulating the TLRs 3, 4 and 7 pathways. Veratric acid significantly down-regulated the protein expression of TLR3 and IRF3 as well as the mRNA expression of TBK1 and TRIF. Vitexin significantly down-regulated the protein expression of TBK1 and IRF3 as well as the mRNA expression of TLR3, TBK1, TRIF and IRF3 while up-regulated the protein expression of TLR4 and IKKalpha. Trolline significantly down-regulated the protein expression of TLR7 whereas significantly up-regulated the protein expression of TLR4, IKKalpha and TAK1. CONCLUSIONS: The three representative compounds from Flos Trollii play their parts in anti-H1N1 viral effect through partially down-regulating TLRs 3 and 7 pathways and up-regulating TLR4 pathway. They counteract the inflammatory injury caused by excessive production of NO, IL-1, IL-6, and TNF-alpha induced by virus infection and enhance the production of IFN-beta so as to eliminate the virus.
A validated UHPLC-MS/MS method for measurement of pharmacokinetics and tissue distribution of trolline in rat.[Pubmed:30877932]
J Pharm Biomed Anal. 2019 May 30;169:208-214.
As a novel alkaloid, Trolline is a potential methionine aminopeptidase inhibitor. However, up to now, no informations about the quantification of Trolline were available in biosamples. In this study, a simple, specific and sensitive analytical method based on UHPLC-MS/MS method has been established and validated for determination of Trolline in rat plasma and tissues after intravenous administration. Sample preparation was carried out by a simple liquid-liquid extraction and carbamazepine was used as internal standard (I.S.). Chromatographic separation was achieved by using a Waters BEH C18 column and involving the optimized mobile phase of 0.1% formic acid aqueous solution and acetonitrile with gradient elution flow of 0.20 ml/min. Trolline and I.S. were detected by multiple reaction monitoring (MRM) modes with positive electrospray ionization and transitions at m/z 220.0-->136.8 for Trolline and m/z 237.0-->193.9 for carbamazepine (I.S.). Good linearity was ranged from 10.0 ng/ml to 4000 ng/ml for Trolline both in plasma and various tissues. The lower limit of quantification (LLOQ) was 10 ng/ml in all samples. The intra- and inter-day precision (RSD%) were below 11.3% and the accuracy (RE%) was ranged from -10.2% to 12.3%. The validated method was successfully applied to the pharmacokinetics and tissue distribution study of Trolline after intravenous administration.
Anti-inflammatory effect of the compounds from the flowers of Trollius chinensis.[Pubmed:30150194]
Pak J Pharm Sci. 2018 Sep;31(5):1951-1957.
In order to investigate the anti-inflammatory activity of flavonoids, phenolic acids, and alkaloids from the flowers of Trollius chinensis, some representative compounds, namely, orientin, 2"-O-beta-L-galactopyranosylorientin, vitexin, quercetin, isoquercetin, luteolin, veratric acid, proglobeflowery acid, trollioside, and Trolline were selected to study their inhibitory effects against LPS-induced NO, IL-6, and TNF-beta release in RAW264.7 cells. At the higher concentration, both phenolic acids and flavonoids inhibited the production of NO, whereas only phenolic acids showed this effect at the lower concentration. Although Trolline had stronger cytotoxicity, it exhibited a potential effect of decreasing NO production induced by LPS in the non-toxic concentration range. In addition, all tested compounds decreased the production of IL-6 and TNF-a by almost 50% at both the higher and lower concentrations. It is concluded that the anti-inflammatory activity of the phenolic acids is stronger than that of the flavonoids.
Alkaloids from Tetrastigma hemsleyanum and Their Anti-Inflammatory Effects on LPS-Induced RAW264.7 Cells.[Pubmed:29899226]
Molecules. 2018 Jun 14;23(6). pii: molecules23061445.
Alkaloids 1(-)10 were isolated from the aerial parts of Tetrastigma hemsleyanum (APTH) and obtained from species of the genus Tetrastigma for the first time. The chemical structures of the isolated compounds were identified by NMR, UV, and MS analyses. Their anti-inflammatory activities were investigated by measuring nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW264.7 macrophages. Among all the isolates, compounds 6, 7 and 10 showed potent inhibitory activity against LPS-stimulated NO production in RAW264.7 cells (IC50: 31.9, 25.2 and 6.3 muM, respectively). Furthermore, APTH and S-(-)-Trolline (10) inhibited induction of inflammatory cytokines or mediators such as interleukin-1beta (IL-1beta) and inducible nitric oxide synthase (iNOS) via suppression of nuclear factor kappaB (NF-kappaB) translocation into the nucleus. In addition, 10 suppressed extracellular signal-regulated protein kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) phosphorylation in a dose-dependent manner. These results conclusively demonstrated that compound 10 displays anti-inflammatory activity via suppression of NF-kappaB activation and the ERK-MAPK signaling pathway in LPS-stimulated RAW264.7 cells.
One-pot chemoenzymatic synthesis of trolline and tetrahydroisoquinoline analogues.[Pubmed:29345260]
Chem Commun (Camb). 2018 Feb 1;54(11):1323-1326.
Chemoenzymatic reaction cascades can provide access to chiral compounds from low-cost starting materials in one pot. Here we describe one-pot asymmetric routes to tetrahydroisoquinoline alkaloids (THIAs) using the Pictet-Spenglerase norcoclaurine synthase (NCS) followed by a cyclisation, to give alkaloids with two new heterocyclic rings. These reactions operated with a high atom economy to generate THIAs in high yields.
Trolline Ameliorates Liver Fibrosis by Inhibiting the NF-kappaB Pathway, Promoting HSC Apoptosis and Suppressing Autophagy.[Pubmed:29141243]
Cell Physiol Biochem. 2017;44(2):436-446.
BACKGROUND/AIMS: Previous studies have shown that Trolline possesses various forms of pharmacological activity, including antibacterial and antiviral potency. The present paper addressed the putative hepatoprotective effects of Trolline. METHODS: Rats received 2 ml/kg CCl4 (mixed 1: 1 in peanut oil) intragastrically twice a week for 8 weeks to induce hepatic fibrosis. The animals were then treated with Trolline for additional 4 weeks. Liver pathology and collagen accumulation were observed by hematoxylin-eosin and Masson's trichrome staining, respectively. Serum transaminase activity and collagen-related indicator level were determined by commercially available kits. NF-kappaB pathway activation was also examined. Moreover, the effects of Trolline on hepatic stellate cell (HSC-T6) apoptosis, mitochondrial membrane potential (MMP), and autophagy were assessed. RESULTS: Trolline significantly alleviated CCl4-induced liver injury and notably reduced the accumulation of collagen in liver tissues. Trolline treatment also markedly decreased inflammatory cytokines levels by inhibiting the NF-kappaB pathway. Trolline strongly inhibited HSC-T6 activation and notably induced cell apoptosis by modulating the Bax/Bcl-2 ratio, caspase activity, and MMP. Moreover, Trolline significantly inhibited HSC-T6 autophagy, as evidenced by the decrease in the formation of autophagic vacuoles and the number of autophagosomes, by regulating the expression levles of LC3, Beclin-1, P62, Atg 5 and 7. CONCLUSION: Our study demonstrates that Trolline ameliorates liver fibrosis, possibly by inhibiting the NF-kappaB pathway, promoting HSCs apoptosis and suppressing autophagy.
Investigation of the effective components of the flowers of Trollius chinensis from the perspectives of intestinal bacterial transformation and intestinal absorption.[Pubmed:28502237]
Pharm Biol. 2017 Dec;55(1):1747-1758.
CONTEXT: The flowers of Trollius chinensis Bunge (Ranunculaceae), used for respiratory tract infections, mainly contain flavonoids, phenolic acids, and alkaloids; however, the effective components are debatable because of their unclear in vivo activities. OBJECTIVE: This study investigates the effective components from the perspectives of biotransformation and absorption. MATERIALS AND METHODS: Both single person derived- and multiple people-derived intestinal florae were used to investigate the biotransformation of aqueous extract of the flowers of T. chinensis (AEOF) at the concentrations of 15.0, 30.0, and 60.0 mg/mL, respectively, for 72 h. Both human colon adenocarcinoma cell line (Caco-2) monolayers and everted gut sacs were employed to evaluate the intestinal absorption of the intestinal bacterial transformed AEOF at the concentrations of 10, 20, and 30 mg/mL, respectively, for 180 min. RESULTS: 2''-O-beta-l-Galactopyranosylorientin, orientin, vitexin, quercetin, veratric acid, proglobeflowery acid, and Trolline in AEOF were not transformed by intestinal bacteria, while isoquercetin and trollioside were completely transformed. The Papp values of 2''-O-beta-l-galactopyranosylorientin, orientin, and vitexin calculated based on the experimental data of intestinal absorption were at the levels of 10(-5), whereas those of veratric acid, proglobeflowery acid, and Trolline were at 10(-4). The mass ratio of flavonoids to phenolic acids to alkaloids changed from 16:10:7 to 9:12:8 before and after absorption. DISCUSSION AND CONCLUSION: The dominant position of flavonoids was replaced by phenolic acids after absorption. In addition to flavonoids which are usually considered as the dominant effective ones, phenolic acids and alkaloids should be also very important for the efficacy of these flowers.
Absorption properties and mechanism of trolline and veratric acid and their implication to an evaluation of the effective components of the flowers of Trollius chinensis.[Pubmed:25263984]
Chin J Nat Med. 2014 Sep;12(9):700-4.
AIM: To study the absorption properties and mechanism of two important components, Trolline and veratric acid, from the flowers of Trollius chinensis, in order to better understand the contribution of these two compounds to the effectiveness of these flowers. METHOD: The human Caco-2 cell monolayer model was employed to study the transport of Trolline and veratric acid from apical side (AP) to basal side (BL), and from BL to AP by determining the transport rates as the function of time and concentration and calculating apparent permeability coefficients (Papp). RESULTS: Trolline and veratric acid were transported across Caco-2 cell monolayer through different mechanisms in a concentration dependent manner. Trolline was transported at a Papp level of 10(-6) cm.s(-1) with a Papp AP-->BL/Papp BL-->AP ratio of more than 1.8 or less than 0.8, while veratric acid was transported at a Papp level of 10(-5)cm.s(-1) with a Papp AP-->BL/Papp BL-->AP ratio of close to 1.0. CONCLUSION: Trolline is moderately absorbed through an associative mechanism involving active and passive transport, and veratric acid is well-absorbed mainly through passive diffusion. These factors should be taken into account when chemically assessing the pharmacodynamic material basis of the flowers of T. chinensis.
[Determination of trolline in flowers of Trollius chinensis by HPLC].[Pubmed:22737861]
Zhongguo Zhong Yao Za Zhi. 2012 Jan;37(2):247-9.
An HPLC method was developed for determinantion of Trolline in the flowers of Trollius chinensis commercially available in China. The HPLC analysis was conducted on an Agilent C18 ODS column (4.6 mm x 250 mm, 5 microm) with acetonitrile-0.5% acetic acid solution (gradient: 0-30 min, 2:98-100:0) as mobile phase at a flow rate of 1.0 mL x min(-1). The detecting wavelength was at 258 nm. Linearity of Trolline was good in the range of 0.1380-1.2410 microg and the content of Trolline in nine batches of the flowers of T. chinensis commercially available in China was ranged from 0.05% to 0.11%. This method is good in terms of precision, accuracy and repeatability and can be used for the quantitative determination of Trolline.
The pyrrolo[2,1-a]isoquinoline alkaloids.[Pubmed:22308756]
Alkaloids Chem Biol. 2011;70:79-151.
The present chapter describes isolation, biogenetic proposals, and syntheses of the natural products 1-4 and 10-11 with a pyrrolo[2,1-a]-isoquinoline framework. Moreover, the syntheses of some structural analogs are discussed. The pyrrolo[2,1-a]isoquinolines are of interest due to their promising biological activities. For crispine A (1), many total syntheses have been reported and for Trolline (3), only three. Only one total synthesis has been reported for each of the following natural products: peyoglutam (10), mescalotam (11), and the antitumor active crispine B (2). Some of the pyrrolo[2,1-a]isoquinoline alkaloids have not been synthesized yet. The following three tables summarize the synthetic efforts toward crispine A (1) (Table 1: racemic syntheses; Table 2: enantioselective syntheses) and Trolline (3) (Table 3).


