BavachalconeCAS# 28448-85-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28448-85-3 | SDF | Download SDF |
| PubChem ID | 6450879 | Appearance | Yellow-orange powder |
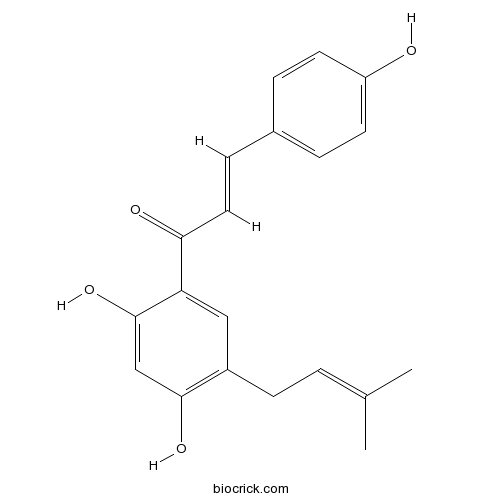
| Formula | C20H20O4 | M.Wt | 324.4 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Synonyms | Broussochalcone B | ||
| Solubility | DMSO : ≥ 34 mg/mL (104.82 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (E)-1-[2,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | CC(=CCC1=C(C=C(C(=C1)C(=O)C=CC2=CC=C(C=C2)O)O)O)C | ||
| Standard InChIKey | BLZGPHNVMRXDCB-UXBLZVDNSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bavachalcone has antibiotic or anticancer activities. 2. Bavachalcone may be useful as a therapeutic drug for bone resorption-associated diseases. 3. Bavachalcone can protect the endothelial function by increasing AMPK activity and MnSOD expression and reducing mitochondrial oxidative stress. |
| Targets | AMPK | SOD | MEK | ERK | Akt | NF-kB |

Bavachalcone Dilution Calculator

Bavachalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0826 mL | 15.4131 mL | 30.8261 mL | 61.6523 mL | 77.0654 mL |
| 5 mM | 0.6165 mL | 3.0826 mL | 6.1652 mL | 12.3305 mL | 15.4131 mL |
| 10 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6165 mL | 1.233 mL | 1.5413 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bavachalcone is a major bioactive compounds isolated from Psoralea corylifolia L.; has been widely used as traditional Chinese medicine; antibiotic or anticancer agent. IC50 value: Target: Bavachalcone inhibited osteoclast formation from precursor cells with the IC(50) of approximately 1.5 microg ml(-1). The activation of MEK, ERK, and Akt by receptor activator of nuclear factor kappaB ligand (RANKL), the osteoclast differentiation factor, was prominently reduced in the presence of bavachalcone. The induction of c-Fos and NFATc1, key transcription factors for osteoclastogenesis, by RANKL was also suppressed by bavachalcone [1]. Bavachalcone exhibited a significant inhibitory effect on baculovirus-expressed BACE-1 in vitro [2]. Bavachalcone had stronger inhibition on UGT1A1 and UGT1A7 than corylin which did not inhibit UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A10, and UGT2B4. Data fitting using Dixon and Lineweaver-Burk plots demonstrated the noncompetitive inhibition of bavachalcone against UGT1A1 and UGT1A7-mediated 4-MU glucuronidation reaction. The values of inhibition kinetic parameters (Ki) were 5.41 μ M and 4.51μ M for UGT1A1 and UGT1A7, respectively [3].
References:
[1]. Park CK, et al. Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL. Biochem Pharmacol. 2008 Jun 1;75(11):2175-82.
[2]. Choi YH, et al. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008 Sep;74(11):1405-8.
[3]. Shan L, et al. Comparison of the Inhibitory Potential of Bavachalcone and Corylin against UDP-Glucuronosyltransferases. Evid Based Complement Alternat Med. 2014;2014:958937.
- 4-(4-Aminophenoxy)-N-methyl-2-pyridinecarboxamide
Catalog No.:BCC8649
CAS No.:284462-37-9
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- 6-Hydroxy-2,6-dimethyl-2,7-octadienoic acid
Catalog No.:BCN1463
CAS No.:28420-25-9
- Ac9-25
Catalog No.:BCC5997
CAS No.:284040-76-2
- NPS-2143
Catalog No.:BCC4409
CAS No.:284035-33-2
- DR 2313
Catalog No.:BCC2451
CAS No.:284028-90-6
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- FR 236924
Catalog No.:BCC7564
CAS No.:28399-31-7
- 1(10)-Aristolen-2-one
Catalog No.:BCN7634
CAS No.:28398-06-3
- Bumetanide
Catalog No.:BCC1119
CAS No.:28395-03-1
- 7-Neohesperidosides
Catalog No.:BCN8200
CAS No.:28383-41-7
- Aloin B
Catalog No.:BCN2576
CAS No.:28371-16-6
- Tomentin
Catalog No.:BCN5180
CAS No.:28449-62-9
- 20-Deacetyltaxuspine X
Catalog No.:BCN7374
CAS No.:284672-76-0
- 9-Deacetyltaxinine E
Catalog No.:BCN7227
CAS No.:284672-78-2
- Delphinidin-3-O-galactoside chloride
Catalog No.:BCN3019
CAS No.:28500-00-7
- Petunidin-3-O-galactoside chloride
Catalog No.:BCN3024
CAS No.:28500-02-9
- Petunidin-3-O-arabinoside chloride
Catalog No.:BCN3026
CAS No.:28500-03-0
- Malvidin-3-O-arabinoside chloride
Catalog No.:BCN3032
CAS No.:28500-04-1
- Anadoline N-oxide
Catalog No.:BCN2029
CAS No.:28513-29-3
- 1-Amino-2-methylpropan-2-ol
Catalog No.:BCN1773
CAS No.:2854-16-2
- BML-190
Catalog No.:BCC4410
CAS No.:2854-32-2
- Theaflavin-3'-gallate
Catalog No.:BCN5421
CAS No.:28543-07-9
- 4-Benzoyloxy-2-azetidinone
Catalog No.:BCC8696
CAS No.:28562-58-5
Comparison of the Inhibitory Potential of Bavachalcone and Corylin against UDP-Glucuronosyltransferases.[Pubmed:24829606]
Evid Based Complement Alternat Med. 2014;2014:958937.
Bavachalcone and corylin are two major bioactive compounds isolated from Psoralea corylifolia L., which has been widely used as traditional Chinese medicine for many years. As two antibiotic or anticancer drugs, Bavachalcone and corylin are used in combination with other drugs; thus it is necessary to evaluate potential pharmacokinetic herb-drug interactions (HDI) of the two bioactive compounds. The aim of the present study was to compare the effects of liver UDP-glucuronosyltransferase (UGT) 1A1, UGT1A3, UGT1A7, UGT1A8, UGT 1A10, and UGT2B4 inhibited by Bavachalcone and corylin. 4-Methylumbelliferone (4-MU) was used as a nonspecific "probe" substrate. Bavachalcone had stronger inhibition on UGT1A1 and UGT1A7 than corylin which did not inhibit UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A10, and UGT2B4. Data fitting using Dixon and Lineweaver-Burk plots demonstrated the noncompetitive inhibition of Bavachalcone against UGT1A1 and UGT1A7-mediated 4-MU glucuronidation reaction. The values of inhibition kinetic parameters (Ki) were 5.41 mu M and 4.51 mu M for UGT1A1 and UGT1A7, respectively. The results of present study suggested that there was a possibility of UGT1A1 and UGT1A7 inhibition-based herb-drug interaction associated with Bavachalcone and provided the basis for further in vivo studies to investigate the HDI potential between Bavachalcone and UGT substrates.
Bavachalcone-induced manganese superoxide dismutase expression through the AMP-activated protein kinase pathway in human endothelial cells.[Pubmed:25766656]
Pharmacology. 2015;95(3-4):105-10.
Mitochondrial oxidative stress has been suggested as a major etiological factor in cardiovascular diseases. Manganese superoxide dismutase (MnSOD) is an essential antioxidant mitochondrial enzyme. Although polyphenols can induce MnSOD expression, their mechanism of action remains unclear. We examined the effect of Bavachalcone, a bioactive compound isolated from Psoralea corylifolia, on MnSOD protein expression and explored whether this effect is mediated through the AMP-activated protein kinase (AMPK) signaling pathway. Our data showed that Bavachalcone enhanced the luciferase activity of the MnSOD promoter and increased MnSOD mRNA and protein expressions. Moreover, Bavachalcone suppressed the mitochondrial superoxide production in endothelial cells. Conversely, Bavachalcone stimulated liver kinase B1 and AMPKalpha phosphorylation. mRNA interference by using short hairpin RNA (shRNA) of AMPK inhibited Bavachalcone-induced MnSOD expression. A-769662, an AMPK activator, also stimulated AMPK activity and increased MnSOD expression. Furthermore, AMPK knockdown by shRNA-AMPK reversed the inhibitory effects of Bavachalcone on mitochondrial superoxide production in endothelial cells. These findings indicate that Bavachalcone can protect the endothelial function by increasing AMPK activity and MnSOD expression and reducing mitochondrial oxidative stress. .
Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL.[Pubmed:18433733]
Biochem Pharmacol. 2008 Jun 1;75(11):2175-82.
Osteoclasts are cells that have a specialized role for bone resorption and are responsible for many bone diseases such as osteoporosis. As herbal products are invaluable sources in discovery of compounds for new therapies, we sought to identify compounds efficacious in suppressing osteoclastogenesis from medicinal plants that have been implicated for treatment of osteoporotic conditions. Bavachalcone was isolated from Psoralea corylifolia, and its effects on osteoclast differentiation were evaluated with primary cultures of osteoclast precursor cells. In addition, the molecular mechanism of action was investigated. Bavachalcone inhibited osteoclast formation from precursor cells with the IC(50) of approximately 1.5 microg ml(-1). The activation of MEK, ERK, and Akt by receptor activator of nuclear factor kappaB ligand (RANKL), the osteoclast differentiation factor, was prominently reduced in the presence of Bavachalcone. The induction of c-Fos and NFATc1, key transcription factors for osteoclastogenesis, by RANKL was also suppressed by Bavachalcone. In conclusion, Bavachalcone inhibits osteoclastogenesis by interfering with the ERK and Akt signaling pathways and the induction of c-Fos and NFATc1 during differentiation. Our results suggest that Bavachalcone may be useful as a therapeutic drug for bone resorption-associated diseases.


