Bacopaside XCAS# 94443-88-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

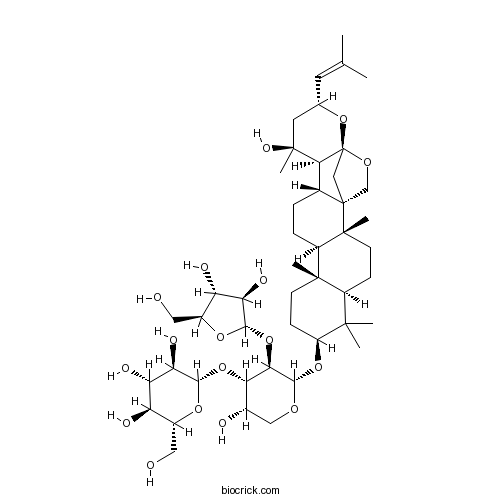
| Cas No. | 94443-88-6 | SDF | Download SDF |
| PubChem ID | 10629555 | Appearance | White powder |
| Formula | C46H74O17 | M.Wt | 899 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Bacopaside VII; Jujubogenin isomer of Bacopasaponin C | ||
| Solubility | Soluble in ethanol and methanol; insoluble in chloroform | ||
| Chemical Name | None | ||
| SMILES | CC(=CC1CC(C2C3CCC4C5(CCC(C(C5CCC4(C36CC2(O1)OC6)C)(C)C)OC7C(C(C(CO7)O)OC8C(C(C(C(O8)CO)O)O)O)OC9C(C(C(O9)CO)O)O)C)(C)O)C | ||
| Standard InChIKey | RANQPHKSRUUPKK-GPUGMLHBSA-N | ||
| Standard InChI | InChI=1S/C46H74O17/c1-21(2)14-22-15-44(7,55)37-23-8-9-28-42(5)12-11-29(41(3,4)27(42)10-13-43(28,6)45(23)19-46(37,63-22)57-20-45)60-40-36(62-38-33(53)31(51)26(17-48)59-38)35(24(49)18-56-40)61-39-34(54)32(52)30(50)25(16-47)58-39/h14,22-40,47-55H,8-13,15-20H2,1-7H3/t22-,23+,24-,25+,26-,27-,28+,29-,30+,31-,32-,33+,34+,35-,36+,37-,38-,39-,40-,42-,43+,44-,45-,46-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bacopaside X (9.06 μM) showed binding affinity towards the D1 receptor. |
| Targets | D1 receptor |
| In vitro | In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri.[Pubmed: 25965066 ]PLoS One. 2015 May 12;10(5):e0126565.Bacopa monnieri has been used in Ayurvedic medicine to improve memory and cognition. The active constituent responsible for its pharmacological effects is bacoside A, a mixture of dammarane-type triterpenoid saponins containing sugar chains linked to a steroid aglycone skeleton. Triterpenoid saponins have been reported to be transformed in vivo to metabolites that give better biological activity and pharmacokinetic characteristics. Thus, the activities of the parent compounds (bacosides), aglycones (jujubogenin and pseudojujubogenin) and their derivatives (ebelin lactone and bacogenin A1) were compared using a combination of in silico and in vitro screening methods.
|
| Structure Identification | Chem Pharm Bull (Tokyo). 2006 Jun;54(6):907-11.Estimation of twelve bacopa saponins in Bacopa monnieri extracts and formulations by high-performance liquid chromatography.[Pubmed: 16755069]
|

Bacopaside X Dilution Calculator

Bacopaside X Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1123 mL | 5.5617 mL | 11.1235 mL | 22.2469 mL | 27.8087 mL |
| 5 mM | 0.2225 mL | 1.1123 mL | 2.2247 mL | 4.4494 mL | 5.5617 mL |
| 10 mM | 0.1112 mL | 0.5562 mL | 1.1123 mL | 2.2247 mL | 2.7809 mL |
| 50 mM | 0.0222 mL | 0.1112 mL | 0.2225 mL | 0.4449 mL | 0.5562 mL |
| 100 mM | 0.0111 mL | 0.0556 mL | 0.1112 mL | 0.2225 mL | 0.2781 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
- JNJ 28871063 hydrochloride
Catalog No.:BCC7662
CAS No.:944342-90-9
- QS 11
Catalog No.:BCC7648
CAS No.:944328-88-5
- A-803467
Catalog No.:BCC5075
CAS No.:944261-79-4
- MJ 15
Catalog No.:BCC7852
CAS No.:944154-76-1
- Peficitinb (ASP015K, JNJ-54781532)
Catalog No.:BCC6503
CAS No.:944118-01-8
- PG 106
Catalog No.:BCC6330
CAS No.:944111-22-2
- Isomartynoside
Catalog No.:BCN4497
CAS No.:94410-22-7
- Tetrachlorohydroquinone dimethyl ether
Catalog No.:BCN1301
CAS No.:944-78-5
- Dimethylmatairesinol
Catalog No.:BCN4496
CAS No.:943989-68-2
- BMS-303141
Catalog No.:BCC4097
CAS No.:943962-47-8
- Ophiopogoside A
Catalog No.:BCC8346
CAS No.:943914-99-6
- Syzalterin
Catalog No.:BCN3969
CAS No.:94451-48-6
- Cromakalim
Catalog No.:BCC7038
CAS No.:94470-67-4
- Lck inhibitor 2
Catalog No.:BCC1690
CAS No.:944795-06-6
- Rhuscholide A
Catalog No.:BCN4498
CAS No.:944804-58-4
- Isodaphnoretin B
Catalog No.:BCN6913
CAS No.:944824-29-7
- Decernotinib(VX-509)
Catalog No.:BCC6456
CAS No.:944842-54-0
- Angelic anhydride
Catalog No.:BCN3411
CAS No.:94487-74-8
- 2'-Acetylacteoside
Catalog No.:BCN3409
CAS No.:94492-24-7
- Tamibarotene
Catalog No.:BCC1983
CAS No.:94497-51-5
- CYM 5541
Catalog No.:BCC6321
CAS No.:945128-26-7
- 7-Prenyljacareubin
Catalog No.:BCN7353
CAS No.:94513-60-7
- 4-Hydroxy-2-methoxyphenol 1-O-(6-O-syringoyl)glucoside
Catalog No.:BCN1300
CAS No.:945259-61-0
Insights into the molecular aspects of neuroprotective Bacoside A and Bacopaside I.[Pubmed:29676230]
Curr Neuropharmacol. 2018 Apr 19. pii: CN-EPUB-89845.
Bacopa monnieri, commonly known as Brahmi, has been extensively used as a neuromedicine for various disorders such as anxiety, depression and memory loss. Chemical characterization studies revealed the major active constituents of the herb as the triterpenoid saponins, bacosides. Bacoside A, the vital neuroprotective constituent, is composed of four constituents viz., bacoside A3, bacopaside II, jujubogenin isomer of bacopasaponin C (Bacopaside X) and bacopasaponin C. B. monnieri extracts as well as bacosides successfully establish a healthy antioxidant environment in various tissues especially in liver and brain. Free radical scavenging, suppression of lipid peroxidation and activation of antioxidant enzymes by bacosides help to attain a physiological state of minimized oxidative stress. The molecular basis of neuroprotective activity of bacosides is attributed to the regulation of mRNA translation and surface expression of neuroreceptors such as AMPAR, NMDAR and GABAR in the various parts of the brain. Bioavailability as well as binding of neuroprotective agents (such as bacosides) to these receptors is controlled by the Blood Brain Barrier (BBB). However, nano conversion of these drug candidates easily resolves the BBB restriction and carries a promising role in future therapies. This review summarizes the neuroprotective functions of the B. monnieri extracts as well as its active compounds (bacoside A, bacopaside I) and the molecular mechanisms responsible for these pharmacological activities.
In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri.[Pubmed:25965066]
PLoS One. 2015 May 12;10(5):e0126565.
Bacopa monnieri has been used in Ayurvedic medicine to improve memory and cognition. The active constituent responsible for its pharmacological effects is bacoside A, a mixture of dammarane-type triterpenoid saponins containing sugar chains linked to a steroid aglycone skeleton. Triterpenoid saponins have been reported to be transformed in vivo to metabolites that give better biological activity and pharmacokinetic characteristics. Thus, the activities of the parent compounds (bacosides), aglycones (jujubogenin and pseudojujubogenin) and their derivatives (ebelin lactone and bacogenin A1) were compared using a combination of in silico and in vitro screening methods. The compounds were docked into 5-HT1A, 5-HT2A, D1, D2, M1 receptors and acetylcholinesterase (AChE) using AutoDock and their central nervous system (CNS) drug-like properties were determined using Discovery Studio molecular properties and ADMET descriptors. The compounds were screened in vitro using radioligand receptor binding and AChE inhibition assays. In silico studies showed that the parent bacosides were not able to dock into the chosen CNS targets and had poor molecular properties as a CNS drug. In contrast, the aglycones and their derivatives showed better binding affinity and good CNS drug-like properties, were well absorbed through the intestines and had good blood brain barrier (BBB) penetration. Among the compounds tested in vitro, ebelin lactone showed binding affinity towards M1 (Ki = 0.45 muM) and 5-HT2A (4.21 muM) receptors. Bacoside A and Bacopaside X (9.06 muM) showed binding affinity towards the D1 receptor. None of the compounds showed any inhibitory activity against AChE. Since the stimulation of M1 and 5-HT2A receptors has been implicated in memory and cognition and ebelin lactone was shown to have the strongest binding energy, highest BBB penetration and binding affinity towards M1 and 5-HT2A receptors, we suggest that B. monnieri constituents may be transformed in vivo to the active form before exerting their pharmacological activity.
An enzyme-linked immunosorbant assay using monoclonal antibody against bacoside A(3) for determination of jujubogenin glycosides in Bacopa monnieri (L.) Wettst.[Pubmed:21413093]
Phytochem Anal. 2011 Sep-Oct;22(5):385-91.
INTRODUCTION: In Ayurvedic medicines, Bacopa monnieri (L.) Wettst. (brahmi) is known as a medicinal plant used for memory enhancement. Its active compounds are classified as pseudojujubogenin and jujubogenin glycosides. Owing to the lack of chromophore in the saponin glycoside structures, HPLC-UV-vis gives low sensitivity for determination of such compounds. In the case of the detection of small amounts of saponin glycosides, immunological assay could be a suitable method. OBJECTIVE: To develop and validate a sensitive enzyme-linked immunosorbant assay (ELISA) using monoclonal antibody (MAb) against bacoside A(3), the major jujubogenin glycoside found in brahmi. METHODOLOGY: An immunogen was prepared by conjugating bacoside A(3) with a bovine serum albumin (BSA). To determine its immunogenicity, the ratio of hapten in bacoside A(3)-BSA conjugate was determined by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF-MS). After immunisation in mice, hybridomas secreting MAbs against bacoside A(3) were produced by fusing the immunised splenocytes with SP2/0- Ag14 myeloma cells. The antibody was raised specifically against jujubogenin glycosides. The ELISA using anti-bacoside A(3) MAb was developed. RESULTS: Bacoside A(3) in the range of 3.05-97.70 ng mL(-)(1) could be detected by ELISA using anti-bacoside A(3) MAb. The assay showed a detection limit of 0.48 ng mL(-)(1) (0.517 nm). The validation study showed that the method was precise, accurate and sensitive. Interestingly, the MAb showed cross-reactivity with the other jujubogenin glycosides, Bacopaside X and IV. However, it did not show cross-reactivity with any of pseudojujubogenin glycosides. CONCLUSION: The study demonstrated that ELISA using anti-bacoside A(3) MAb can be used for determination of total jujubogenin glycosides in brahmi.
Silica-based monolithic column with evaporative light scattering detector for HPLC analysis of bacosides and apigenin in Bacopa monnieri.[Pubmed:19606439]
J Sep Sci. 2009 Aug;32(15-16):2812-8.
A high performance liquid chromatographic method using a silica-based monolithic column coupled with evaporative light scattering detector (HPLC-ELSD) was developed and validated for simultaneous quantification of bacosides (bacoside A, bacopaside I, bacoside A(3), bacopaside II, Bacopaside X, bacopasaponin C) and apigenin in Bacopa monnieri. The chromatographic resolution was achieved on a Chromolith RP-18 (100x4.6 mm) column with acetonitrile/water (30:70) as mobile phase in isocratic elution at a flow rate of 0.7 mL/min. The drift tube temperature of the ELSD was set to 95 degrees C, and the nitrogen flow rate was 2.0 SLM (standard liter per minute). The calibration curves revealed a good linear relationship (r(2) > 0.9988) within the test ranges. The detection limits (S/N = 3) and the quantification limits (S/N = 10) for the compounds were in the range of 0.54-6.06 and 1.61-18.78 microg/mL, respectively. Satisfactory average recovery was observed in the range of 95.8-99.0%. The method showed good reproducibility for the quantification of these compounds in B. monnieri with intra- and inter-day precision of less than 0.69 and 0.67%, respectively. The validated method was successfully applied to quantify analytes in nine accessions of B. monnieri and thus provides a new basis for overall quality assessment of B. monnieri.
Estimation of twelve bacopa saponins in Bacopa monnieri extracts and formulations by high-performance liquid chromatography.[Pubmed:16755069]
Chem Pharm Bull (Tokyo). 2006 Jun;54(6):907-11.
A simple and sensitive reversed phase high performance liquid chromatographic (HPLC) method has been developed for the simultaneous determination of twelve bacopa saponins present in the extracts of the Indian Medicinal Plant, Bacopa monnieri. The separation was achieved on a reversed phase C(18) column (Luna C(18)), 5 microm by isocratic elution with 0.05 M sodium sulphate buffer (pH 2.3) and acetonitrile (68.5 : 31.5, v/v) as the mobile phase at a flow rate of 1.0 ml/min with an operating temperature of 30 degrees C. The method was validated for linearity, precision, intra- and inter-day precision and accuracy. Several Bacopa samples (plant materials, extracts and commercial formulations) were successfully analyzed. Major bacopasaponins were bacosides A(3) (3), bacopaside II (4), bacopaside I (5), Bacopaside X (6), bacopasaponin C (7), bacopaside N2 (9) and the minor components were bacopasaponin F (1), bacopasaponin E (2), bacopaside N1 (8) bacopaside III (10), bacopaside IV (11) and bacopaside V (12). The total saponin content in the samples, plant materials and extracts varied from 5.1 to 22.17% and 1.47 to 66.03 mg/capsule or tablet in the commercial formulations.


