AvobenzoneCAS# 70356-09-1 |
- RN486
Catalog No.:BCC3921
CAS No.:1242156-23-5
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70356-09-1 | SDF | Download SDF |
| PubChem ID | 51040 | Appearance | Powder |
| Formula | C20H22O3 | M.Wt | 310.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (161.09 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
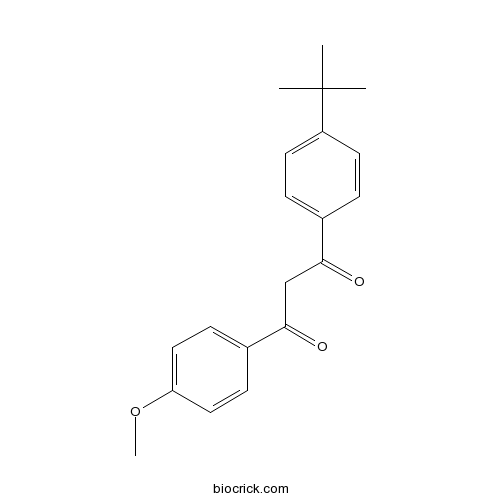
| Chemical Name | 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione | ||
| SMILES | CC(C)(C)C1=CC=C(C=C1)C(=O)CC(=O)C2=CC=C(C=C2)OC | ||
| Standard InChIKey | XNEFYCZVKIDDMS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22O3/c1-20(2,3)16-9-5-14(6-10-16)18(21)13-19(22)15-7-11-17(23-4)12-8-15/h5-12H,13H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Avobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative.
Target: Others
Avobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative. It can degrade faster in light in combination with mineral UV absorbers like zinc oxide and titanium dioxide, though with the right coating of the mineral particles this reaction can be reduced. A manganese doped titanium dioxide may be better than undoped titanium dioxide to improve avobenzone's stability. It reacts with minerals to form colored complexes. Manufacturers of avobenzone, like DSM recommend to include a chelator to prevent this from happening. They also recommend to avoid the inclusion of iron and ferric salts, heavy metals, formaldehyde donors and PABA and PABA esters[1]. References: | |||||

Avobenzone Dilution Calculator

Avobenzone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2218 mL | 16.1088 mL | 32.2175 mL | 64.4351 mL | 80.5438 mL |
| 5 mM | 0.6444 mL | 3.2218 mL | 6.4435 mL | 12.887 mL | 16.1088 mL |
| 10 mM | 0.3222 mL | 1.6109 mL | 3.2218 mL | 6.4435 mL | 8.0544 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2887 mL | 1.6109 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6444 mL | 0.8054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Avobenzone
- Methyl Kakuol
Catalog No.:BCN8243
CAS No.:70342-29-9
- 3-Benzoylthiazolidine-2-thione
Catalog No.:BCC8624
CAS No.:70326-37-3
- Pertussis Toxin
Catalog No.:BCC7565
CAS No.:70323-44-3
- Fluoroorotic Acid, Ultra Pure
Catalog No.:BCC1209
CAS No.:703-95-7
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- CP 775146
Catalog No.:BCC7881
CAS No.:702680-17-9
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- (±)-Lauroylcarnitine chloride
Catalog No.:BCC6690
CAS No.:7023-03-2
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- 3-Aminoadamantan-1-ol
Catalog No.:BCC8618
CAS No.:702-82-9
- Sideritoflavone
Catalog No.:BCN6689
CAS No.:70360-12-2
- Lornoxicam
Catalog No.:BCC4425
CAS No.:70374-39-9
- 6alpha-Hydroxynidorellol
Catalog No.:BCN4598
CAS No.:70387-38-1
- Voleneol
Catalog No.:BCN4266
CAS No.:70389-88-7
- 4(15),11-Oppositadien-1-ol
Catalog No.:BCN4267
CAS No.:70389-96-7
- Dihydrotamarixetin
Catalog No.:BCN4268
CAS No.:70411-27-7
- 17alpha-Neriifolin
Catalog No.:BCN4269
CAS No.:7044-31-7
- (+)-MK 801
Catalog No.:BCC1288
CAS No.:70449-94-4
- Pefloxacin
Catalog No.:BCC4231
CAS No.:70458-92-3
- Pefloxacin Mesylate
Catalog No.:BCC4821
CAS No.:70458-95-6
- Norfloxacin
Catalog No.:BCC4688
CAS No.:70458-96-7
- Corymbol
Catalog No.:BCN6617
CAS No.:7047-54-3
Optimization and Validation of a Fast UPLC Method for Simultaneous Determination of Hydroquinone, Kojic Acid, Octinoxate, Avobenzone, BHA, and BHT.[Pubmed:27874826]
J AOAC Int. 2017 Jan 1;100(1):1-7.
A previously published HPLC method for the simultaneous determination of six major components (hydroquinone, kojic acid, octinoxate, Avobenzone, butylated hydroxyanisole, and butylated hydroxytoluene) in a skin-whitening cream was transferred and optimized to an ultra-performance LC system. Separation was achieved in a ZORBAX SB-Phenyl Rapid-Resolution High Throughput column (2.1 x 100 mm, 1.8 mum), using a mobile phase consisting of water with 0.1% acetic acid and acetonitrile at a flow rate of 0.7 mL/min. The column was maintained at 40 degrees C, and detection was carried out at 230 nm using a diode-array detector. These chromatographic conditions allow the separation of the six compounds in 3 min instead of 14 min. The extraction procedure was optimized, reducing the time and demonstrating its suitability. The method was validated according to International Conference on Harmonization guidelines, with respect to specificity, precision, accuracy, and linearity. Selectivity was found to be satisfactory. Linear regression analysis data for all compounds showed a good linear relationship, with r2 > 0.999 in the concentration range of 50-120% of the label claim for each compound. The RSD for precision and accuracy of the method was found to be less than 2% for all compounds. Comparison of system performance with the previously published HPLC method was made with respect to analysis time, efficacy, and resolution. The proposed method is faster and consumes less solvent and was applied in the determination of six major compounds in batches of skin-whitening cream manufactured during the validation process.
A new insight into the photochemistry of avobenzone in gas phase and acetonitrile from ab initio calculations.[Pubmed:27443629]
Phys Chem Chem Phys. 2016 Aug 10;18(32):22168-78.
Avobenzone (4-tert-butyl-4'-methoxydibenzoylmethane, AB) is one of the most widely used filters in sunscreens for skin photoprotection in the UVA band. The photochemistry of AB includes keto-enol tautomerization, cis-trans isomerization, rotation about the single bond and alpha bond cleavages of carbonyl groups. In this contribution we study chelated and non-chelated enol, rotamers Z and E, and keto tautomers of AB in the ground and excited states in gas phase and acetonitrile by means of a coupled cluster method. Our findings suggest that torsion around the double C2-C3 bond of photoexcited chelated enol leads to internal conversion to the ground state and formation of rotamer E. In addition, opening of the chelated hydrogen ring by torsion of the hydroxyl group creates non-chelated enol. The possible mechanisms of rotamer Z formation are discussed. The solvent dependent photolability is related to the relative order of the lowest triplet pipi* and npi* states of the keto tautomer.
Single- and mixture toxicity of three organic UV-filters, ethylhexyl methoxycinnamate, octocrylene, and avobenzone on Daphnia magna.[Pubmed:27915143]
Ecotoxicol Environ Saf. 2017 Mar;137:57-63.
In freshwater environments, aquatic organisms are generally exposed to mixtures of various chemical substances. In this study, we tested the toxicity of three organic UV-filters (ethylhexyl methoxycinnamate, octocrylene, and Avobenzone) to Daphnia magna in order to evaluate the combined toxicity of these substances when in they occur in a mixture. The values of effective concentrations (ECx) for each UV-filter were calculated by concentration-response curves; concentration-combinations of three different UV-filters in a mixture were determined by the fraction of components based on EC25 values predicted by concentration addition (CA) model. The interaction between the UV-filters were also assessed by model deviation ratio (MDR) using observed and predicted toxicity values obtained from mixture-exposure tests and CA model. The results from this study indicated that observed ECxmix (e.g., EC10mix, EC25mix, or EC50mix) values obtained from mixture-exposure tests were higher than predicted ECxmix (e.g., EC10mix, EC25mix, or EC50mix) values calculated by CA model. MDR values were also less than a factor of 1.0 in a mixtures of three different UV-filters. Based on these results, we suggest for the first time a reduction of toxic effects in the mixtures of three UV-filters, caused by antagonistic action of the components. Our findings from this study will provide important information for hazard or risk assessment of organic UV-filters, when they existed together in the aquatic environment. To better understand the mixture toxicity and the interaction of components in a mixture, further studies for various combinations of mixture components are also required.
Diethylamino hydroxybenzoyl hexyl benzoate (DHHB) as additive to the UV filter avobenzone in cosmetic sunscreen formulations - Evaluation of the photochemical behavior and photostabilizing effect.[Pubmed:28042100]
Eur J Pharm Sci. 2017 Mar 1;99:299-309.
The aim of the present study was to investigate the photochemical behavior of DHHB and its photostabilizing effect on Avobenzone (AVO) in different sunscreen formulations. The formulations were subjected to photostability studies by HPLC and spectrophotometry. In vitro phototoxicity was assessed using 3T3 fibroblast cultures. The mechanism of interaction between DHHB and AVO was investigated by steady state and time-resolved fluorescence spectroscopy. All formulations provided ultra-protection against UVA radiation. HPLC results demonstrated that DHHB did not present a photostabilizing effect on AVO. Fluorescence spectroscopy showed that AVO and DHHB interact by a static quenching mechanism and DHHB did not affect the AVO excited state lifetime. In addition, the energy transfer by Forster mechanism (FRET), which is the most often mechanism responsible for singlet-singlet quenching, is unlikely in this work. These results suggest why DHHB did not work as a photostabilizer on AVO singlet excited state. Phototoxicity results demonstrated that combinations containing DHHB (C2) did not show a phototoxic potential. Finally, although DHHB was considered to be photostable for all formulations studied (F2 and F3) it did not increase the photostability of AVO (F3). Thus, we suggested that formulations containing DHHB (F2) should be considered more advantageous than formulations containing AVO and AVO/DHHB (F1 and F3 respectively).


