SideritoflavoneCAS# 70360-12-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70360-12-2 | SDF | Download SDF |
| PubChem ID | 155493 | Appearance | Yellow powder |
| Formula | C18H16O8 | M.Wt | 360.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
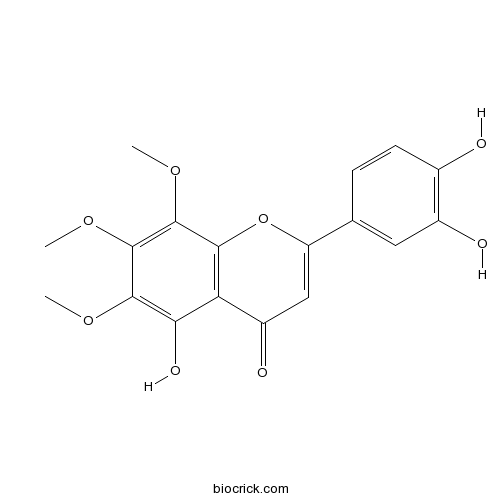
| Chemical Name | 2-(3,4-dihydroxyphenyl)-5-hydroxy-6,7,8-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C2=C(C(=C1OC)OC)OC(=CC2=O)C3=CC(=C(C=C3)O)O)O | ||
| Standard InChIKey | UWNUJPINKMRKKR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O8/c1-23-16-14(22)13-11(21)7-12(8-4-5-9(19)10(20)6-8)26-15(13)17(24-2)18(16)25-3/h4-7,19-20,22H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sideritoflavone has anti-inflammatory effect , it is a selective inhibitor of lipoxygenase activity in vitro. |
| Targets | PGE | Opioid Receptor |

Sideritoflavone Dilution Calculator

Sideritoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7755 mL | 13.8773 mL | 27.7546 mL | 55.5093 mL | 69.3866 mL |
| 5 mM | 0.5551 mL | 2.7755 mL | 5.5509 mL | 11.1019 mL | 13.8773 mL |
| 10 mM | 0.2775 mL | 1.3877 mL | 2.7755 mL | 5.5509 mL | 6.9387 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5551 mL | 1.1102 mL | 1.3877 mL |
| 100 mM | 0.0278 mL | 0.1388 mL | 0.2775 mL | 0.5551 mL | 0.6939 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Avobenzone
Catalog No.:BCC4891
CAS No.:70356-09-1
- Methyl Kakuol
Catalog No.:BCN8243
CAS No.:70342-29-9
- 3-Benzoylthiazolidine-2-thione
Catalog No.:BCC8624
CAS No.:70326-37-3
- Pertussis Toxin
Catalog No.:BCC7565
CAS No.:70323-44-3
- Fluoroorotic Acid, Ultra Pure
Catalog No.:BCC1209
CAS No.:703-95-7
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- CP 775146
Catalog No.:BCC7881
CAS No.:702680-17-9
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- (±)-Lauroylcarnitine chloride
Catalog No.:BCC6690
CAS No.:7023-03-2
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- Lornoxicam
Catalog No.:BCC4425
CAS No.:70374-39-9
- 6alpha-Hydroxynidorellol
Catalog No.:BCN4598
CAS No.:70387-38-1
- Voleneol
Catalog No.:BCN4266
CAS No.:70389-88-7
- 4(15),11-Oppositadien-1-ol
Catalog No.:BCN4267
CAS No.:70389-96-7
- Dihydrotamarixetin
Catalog No.:BCN4268
CAS No.:70411-27-7
- 17alpha-Neriifolin
Catalog No.:BCN4269
CAS No.:7044-31-7
- (+)-MK 801
Catalog No.:BCC1288
CAS No.:70449-94-4
- Pefloxacin
Catalog No.:BCC4231
CAS No.:70458-92-3
- Pefloxacin Mesylate
Catalog No.:BCC4821
CAS No.:70458-95-6
- Norfloxacin
Catalog No.:BCC4688
CAS No.:70458-96-7
- Corymbol
Catalog No.:BCN6617
CAS No.:7047-54-3
- Schizanthine A
Catalog No.:BCN1936
CAS No.:70474-24-7
Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids.[Pubmed:1650522]
Agents Actions. 1991 Mar;32(3-4):283-8.
A group of flavonoids isolated from medicinal plants and which are selective inhibitors of lipoxygenase activity in vitro: Sideritoflavone, cirsiliol, hypolaetin-8-O-beta-D-glucoside, hypolaetin, oroxindin, quercetagetin-7-O-beta-D-glucoside, gossypin, hibifolin and gossypetin, besides leucocyanidol, have been studied for their effects on acute responses induced by carrageenin in mice. The oral administration of flavonoids to mice inhibited dose-dependently the development of paw oedema at 1, 3 and 5 h after carrageenin injection. A similar administration of flavonoids induced a dose-dependent inhibition of leukocyte accumulation in inflammatory exudates following intraperitoneal injection of carrageenin into mice. Some of the flavonoids exhibited a potency against leukocyte infiltration similar to that seen for inhibition of carrageenin oedema at 3 h of induction. In agreement with data reported in rats, indomethacin was much more effective on inhibition of prostaglandin E2 (PGE2) formation than on leukocyte infiltration in mice. The selectivity of flavonoids towards lipoxygenase is not retained in vivo since they behave as dual inhibitors of PGE2 and leukotriene B4 (LTB4) formation in peritoneal exudates. Our data support the inhibition of arachidonic acid metabolism as one of the mechanisms by which flavonoids exert their anti-inflammatory effects.
Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: in silico, in vitro, and in vivo studies.[Pubmed:25562563]
J Nat Prod. 2015 Jan 23;78(1):69-76.
Fractionation of the bioactive dichloromethane extract from the aerial parts of Stachys glutinosa led to the isolation of four flavones, xanthomicrol (1), Sideritoflavone (2), 8-methoxycirsilineol (3), and eupatilin (4), along with two neo-clerodane diterpenes, roseostachenone (8) and a new compound, 3alpha,4alpha-epoxyroseostachenol (7). In order to study structure-activity relationships, two methoxyflavones [5-demethyltangeretin (5) and tangeretin (6)] were synthesized by the methoxylation of xanthomicrol. The isolated compounds (1-4, 7, and 8) as well as the xanthomicrol semisynthetic derivatives (5 and 6) were evaluated for their binding affinity to the mu and delta opioid receptors. Xanthomicrol was the most potent binder to both mu and delta receptors, with a Ki value of 0.83 and 3.6 muM, respectively. Xanthomicrol administered intraperitoneally in mice at a dose of 80 mg/kg significantly reduced morphine-induced antinociception in the tail flick test. Our results suggested that xanthomicrol is a mu opioid receptor antagonist. Docking experiments were carried out to acquire a deeper understanding about important structural aspects of binding of xanthomicrol. In summary, these data suggest that xanthomicrol is a valuable structure for further development into a potential mu opioid receptor antagonist.


