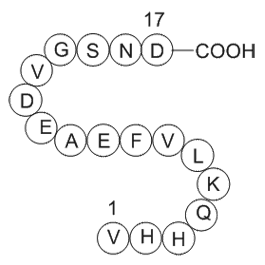
Amyloid Beta-Peptide (12-28) (human)sequence H2N-VHHQKLVFFAEDVGSNK-OH CAS# 107015-83-8 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107015-83-8 | SDF | Download SDF |
| PubChem ID | 90488721 | Appearance | Powder |
| Formula | C89H135N25O25 | M.Wt | 1955.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.70 mg/ml in water | ||
| Sequence | VHHQKLVFFAEDVGSNK | ||
| SMILES | CC(C)CC(C(=O)NC(C(C)C)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(CC2=CC=CC=C2)C(=O)NC(C)C(=O)NC(CCC(=O)O)C(=O)NC(CC(=O)O)C(=O)NC(C(C)C)C(=O)NCC(=O)NC(CO)C(=O)NC(CC(=O)N)C(=O)NC(CCCCN)C(=O)O)NC(=O)C(CCCCN)NC(=O)C(CCC(=O)N)NC(=O)C(CC3=CNC=N3)NC(=O)C(CC4=CNC=N4)NC(=O)C(C(C)C)N | ||
| Standard InChIKey | WOMPQPMHRDDZMP-IJBWYZRXSA-N | ||
| Standard InChI | InChI=1S/C89H135N25O25/c1-45(2)32-58(106-75(124)54(24-16-18-30-90)103-76(125)55(26-28-66(92)116)104-80(129)61(35-52-39-95-43-98-52)108-81(130)62(36-53-40-96-44-99-53)111-86(135)71(94)46(3)4)83(132)114-73(48(7)8)88(137)112-60(34-51-22-14-11-15-23-51)79(128)107-59(33-50-20-12-10-13-21-50)78(127)100-49(9)74(123)102-56(27-29-69(119)120)77(126)110-64(38-70(121)122)84(133)113-72(47(5)6)87(136)97-41-68(118)101-65(42-115)85(134)109-63(37-67(93)117)82(131)105-57(89(138)139)25-17-19-31-91/h10-15,20-23,39-40,43-49,54-65,71-73,115H,16-19,24-38,41-42,90-91,94H2,1-9H3,(H2,92,116)(H2,93,117)(H,95,98)(H,96,99)(H,97,136)(H,100,127)(H,101,118)(H,102,123)(H,103,125)(H,104,129)(H,105,131)(H,106,124)(H,107,128)(H,108,130)(H,109,134)(H,110,126)(H,111,135)(H,112,137)(H,113,133)(H,114,132)(H,119,120)(H,121,122)(H,138,139)/t49-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,71-,72-,73-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amyloid β-peptide fragment; minimum section required to bind to brain proteins. Binds with high affinity to α7-nicotinic ACh receptors, and impairs memory retention following central administration in mice in vivo. |

Amyloid Beta-Peptide (12-28) (human) Dilution Calculator

Amyloid Beta-Peptide (12-28) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amyloid beta (Aβ or Abeta) is a peptide of 36–43 amino acids that is processed from the Amyloid precursor protein. While best known as a component of amyloid plaques in association with Alzheimer's disease, evidence has been found that Aβ is a highly multifunctional peptide with significant non-pathological activity(1). Aβ is the main component of deposits found in the brains of patients with Alzheimer's disease. Brain Aβ is elevated in patients with sporadic Alzheimer’s disease. Aβ is the main constituent of brain parenchymal and vascular amyloid, it contributes to cerebrovascular lesions and is neurotoxic(2).
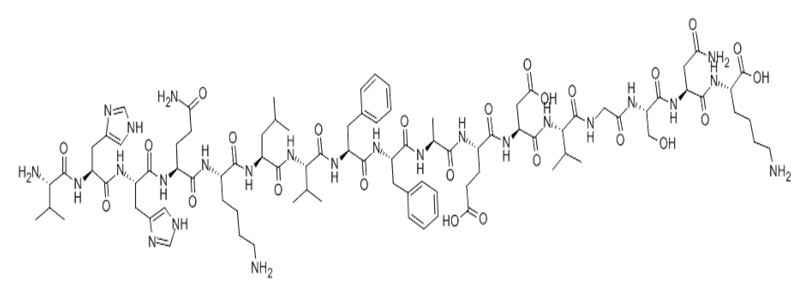
Figure1 Formula of Amyloid β-Peptide (12-28) (human)
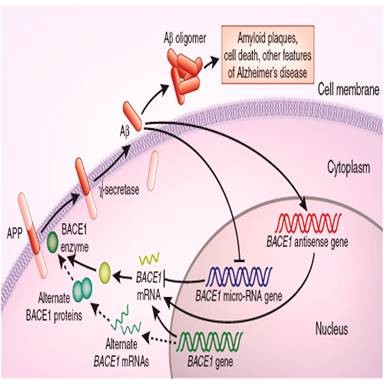
Figure2 signal pathway of Amyloid β
Ref:
1. Lahiri DK, Maloney B (September 2010). "Beyond the signaling effect role of amyloid–β42 on the processing of AβPP, and its clinical implications". Exp. Neurol. 225 (1): 51–4.
2. Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M (September 1998). "Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau". Nat. Neurosci. 1 (5): 355–8.
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- Sarcosine
Catalog No.:BCN2744
CAS No.:107-97-1
- H-ß-Ala-OH
Catalog No.:BCC2851
CAS No.:107-95-9
- 3-Methyl-1-butylamine
Catalog No.:BCN1810
CAS No.:107-85-7
- N-Methyltaurine
Catalog No.:BCN1751
CAS No.:107-68-6
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
- Taurine
Catalog No.:BCN1750
CAS No.:107-35-7
- Propylamine
Catalog No.:BCN1814
CAS No.:107-10-8
- Boc-isoleucinol
Catalog No.:BCC3096
CAS No.:106946-74-1
- Adefovir
Catalog No.:BCC8808
CAS No.:106941-25-7
- Boc-D-Leucinol
Catalog No.:BCC2723
CAS No.:106930-51-2
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- O-Phosphorylethanolamine
Catalog No.:BCN1759
CAS No.:1071-23-4
- EIT hydrobromide
Catalog No.:BCC6824
CAS No.:1071-37-0
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
Abeta association inhibition by transferrin.[Pubmed:23870268]
Biophys J. 2013 Jul 16;105(2):473-80.
The iron-transport glycoprotein transferrin has recently been shown to serve as a potent inhibitor of Abeta self-association. Although this novel, to our knowledge, inhibitory function of transferrin is of potential therapeutic interest for the treatment of Alzheimer's disease, the underlying mechanism is still not fully understood. Although it has been shown that the Fe(III) sequestration by transferrin reduces oxidative damage and Abeta aggregation, it is not clear whether transferrin is also able to inhibit Abeta self-association through direct binding of Abeta. Here, using saturation transfer and off-resonance relaxation NMR spectroscopy, we show that transferrin inhibits Abeta aggregation also by preferentially binding Abeta oligomers and outcompeting Abeta monomers that would otherwise cause the growth of the Abeta oligomers into larger assemblies. This inhibitory mechanism is different from the iron-sequestration model, but it is qualitatively similar to a mechanism previously proposed for the inhibition of Abeta self-association by another plasma and cerebrospinal fluid protein, i.e., human serum albumin. These results suggest that Abeta monomer competition through direct Abeta oligomer binding might be a general strategy adopted by proteins in plasma and cerebrospinal fluid to prevent Abeta aggregation.
Insights on the Interaction between Transthyretin and Abeta in Solution. A Saturation Transfer Difference (STD) NMR Analysis of the Role of Iododiflunisal.[Pubmed:28587455]
J Med Chem. 2017 Jul 13;60(13):5749-5758.
Several strategies against Alzheimer disease (AD) are directed to target Abeta-peptides. The ability of transthyretin (TTR) to bind Abeta-peptides and the positive effect exerted by some TTR stabilizers for modulating the TTR-Abeta interaction have been previously studied. Herein, key structural features of the interaction between TTR and the Abeta(12-28) peptide (3), the essential recognition element of Abeta, have been unravelled by STD-NMR spectroscopy methods in solution. Molecular aspects related to the role of the TTR stabilizer iododiflunisal (IDIF, 5) on the TTR-Abeta complex have been also examined. The NMR results, assisted by molecular modeling protocols, have provided a structural model for the TTR-Abeta interaction, as well as for the ternary complex formed in the presence of IDIF. This basic structural information could be relevant for providing light on the mechanisms involved in the ameliorating effects of AD symptoms observed in AD/TTR(+/-) animal models after IDIF treatment and eventually for designing new molecules toward AD therapeutic drugs.
Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis.[Pubmed:25988463]
JAMA. 2015 May 19;313(19):1939-49.
IMPORTANCE: Amyloid-beta positron emission tomography (PET) imaging allows in vivo detection of fibrillar plaques, a core neuropathological feature of Alzheimer disease (AD). Its diagnostic utility is still unclear because amyloid plaques also occur in patients with non-AD dementia. OBJECTIVE: To use individual participant data meta-analysis to estimate the prevalence of amyloid positivity on PET in a wide variety of dementia syndromes. DATA SOURCES: The MEDLINE and Web of Science databases were searched from January 2004 to April 2015 for amyloid PET studies. STUDY SELECTION: Case reports and studies on neurological or psychiatric diseases other than dementia were excluded. Corresponding authors of eligible cohorts were invited to provide individual participant data. DATA EXTRACTION AND SYNTHESIS: Data were provided for 1359 participants with clinically diagnosed AD and 538 participants with non-AD dementia. The reference groups were 1849 healthy control participants (based on amyloid PET) and an independent sample of 1369 AD participants (based on autopsy). MAIN OUTCOMES AND MEASURES: Estimated prevalence of positive amyloid PET scans according to diagnosis, age, and apolipoprotein E (APOE) epsilon4 status, using the generalized estimating equations method. RESULTS: The likelihood of amyloid positivity was associated with age and APOE epsilon4 status. In AD dementia, the prevalence of amyloid positivity decreased from age 50 to 90 years in APOE epsilon4 noncarriers (86% [95% CI, 73%-94%] at 50 years to 68% [95% CI, 57%-77%] at 90 years; n = 377) and to a lesser degree in APOE epsilon4 carriers (97% [95% CI, 92%-99%] at 50 years to 90% [95% CI, 83%-94%] at 90 years; n = 593; P < .01). Similar associations of age and APOE epsilon4 with amyloid positivity were observed in participants with AD dementia at autopsy. In most non-AD dementias, amyloid positivity increased with both age (from 60 to 80 years) and APOE epsilon4 carriership (dementia with Lewy bodies: carriers [n = 16], 63% [95% CI, 48%-80%] at 60 years to 83% [95% CI, 67%-92%] at 80 years; noncarriers [n = 18], 29% [95% CI, 15%-50%] at 60 years to 54% [95% CI, 30%-77%] at 80 years; frontotemporal dementia: carriers [n = 48], 19% [95% CI, 12%-28%] at 60 years to 43% [95% CI, 35%-50%] at 80 years; noncarriers [n = 160], 5% [95% CI, 3%-8%] at 60 years to 14% [95% CI, 11%-18%] at 80 years; vascular dementia: carriers [n = 30], 25% [95% CI, 9%-52%] at 60 years to 64% [95% CI, 49%-77%] at 80 years; noncarriers [n = 77], 7% [95% CI, 3%-18%] at 60 years to 29% [95% CI, 17%-43%] at 80 years. CONCLUSIONS AND RELEVANCE: Among participants with dementia, the prevalence of amyloid positivity was associated with clinical diagnosis, age, and APOE genotype. These findings indicate the potential clinical utility of amyloid imaging for differential diagnosis in early-onset dementia and to support the clinical diagnosis of participants with AD dementia and noncarrier APOE epsilon4 status who are older than 70 years.
Gas-phase structure of amyloid-beta (12-28) peptide investigated by infrared spectroscopy, electron capture dissociation and ion mobility mass spectrometry.[Pubmed:24043520]
J Am Soc Mass Spectrom. 2013 Dec;24(12):1937-49.
The gas-phase structures of doubly and triply protonated Amyloid-beta12-28 peptides have been investigated through the combination of ion mobility (IM), electron capture dissociation (ECD) mass spectrometry, and infrared multi-photon dissociation (IRMPD) spectroscopy together with theoretical modeling. Replica-exchange molecular dynamics simulations were conducted to explore the conformational space of these protonated peptides, from which several classes of structures were found. Among the low-lying conformers, those with predicted diffusion cross-sections consistent with the ion mobility experiment were further selected and their IR spectra simulated using a hybrid quantum mechanical/semiempirical method at the ONIOM DFT/B3LYP/6-31 g(d)/AM1 level. In ECD mass spectrometry, the c/z product ion abundance (PIA) has been analyzed for the two charge states and revealed drastic differences. For the doubly protonated species, N - Calpha bond cleavage occurs only on the N and C terminal parts, while a periodic distribution of PIA is clearly observed for the triply charged peptides. These PIA distributions have been rationalized by comparison with the inverse of the distances from the protonated sites to the carbonyl oxygens for the conformations suggested from IR and IM experiments. Structural assignment for the amyloid peptide is then made possible by the combination of these three experimental techniques that provide complementary information on the possible secondary structure adopted by peptides. Although globular conformations are favored for the doubly protonated peptide, incrementing the charge state leads to a conformational transition towards extended structures with 310- and alpha-helix motifs.
Nicotine enhances the depressive actions of A beta 1-40 on long-term potentiation in the rat hippocampal CA1 region in vivo.[Pubmed:12611941]
J Neurophysiol. 2003 Jun;89(6):2917-22.
Hippocampal long-term potentiation (LTP) is a form of synaptic plasticity used as a cellular model of memory. Beta amyloid (A beta) is involved in Alzheimer's disease (AD), a neurode-generative disorder leading to cognitive deficits. Nicotine is also claimed to act as a cognitive enhancer. A beta is known to bind with high affinity to the alpha 7-nicotinic acetylcholine receptor (nAChR). Here we have investigated the effect of intracerebroventricular (i.c.v.) injection of the endogenous peptide A beta 1-40 on LTP in area CA1 of urethananesthetized rats. We also examined the effect of A beta 12-28 (i.c.v.), which binds with high affinity to the alpha 7-nAChR and the specific alpha 7-nAChR antagonist methyllycaconitine (MLA) on LTP. We found that A beta 12-28 had no effect on LTP, whereas MLA depressed significantly LTP, suggesting that activation of the alpha 7-nAChR is a requirement for LTP. Within the in vivo environment, where other factors may compete with A beta 12-28 for binding to alpha 7-nAChR, it does not appear to modulate LTP. To determine if the depressive action of A beta 1-40 on LTP could be modulated by nicotine, these agents were also co-applied. Injection of 1 or 10 nmol A beta 1-40 caused a significant depression of LTP, whereas nicotine alone (3 mg/kg) had no effect on LTP. Co-injection of nicotine with A beta 1-40 1 h prior to LTP induction caused a further significant depression of LTP compared with A beta 1-40 alone. These results demonstrate that nicotine enhances the deficit in LTP produced by A beta 1-40. This then suggests that nicotine may exacerbate the depressive actions of A beta on synaptic plasticity in AD.
Binding of amyloid beta-protein to intracellular brain proteins in rat and human.[Pubmed:9804283]
Neurochem Res. 1998 Oct;23(10):1277-82.
Amyloid beta-protein (Abeta), in its soluble form, is known to bind several circulatory proteins such as apolipoprotein (apo) E, apo J and transthyretin. However, the binding of Abeta to intracellular proteins has not been studied. We have developed an overlay assay to study Abeta binding to intracellular brain proteins. The supernatants from both rat and human brains were found to contain several proteins that bind to Abeta 1-40 and Abeta 1-42. No major difference was observed in the Abeta binding-proteins from brain supernatants of patients with Alzheimer's disease and normal age-matched controls. Binding studies using shorter amyloid beta-peptides and competitive overlay assays showed that the binding site of Abeta to brain proteins resides between 12-28 amino acid sequence of Abeta. The presence of several intracellular Abeta-binding (AbetaB) proteins suggests that these proteins may either protect Abeta from its fibrillization or alternatively promote Abeta polymerization. Identification of these proteins and their binding affinities for Abeta are needed to assess their potential role in the pathogenesis of Alzheimer's disease.
An amyloid beta-protein fragment, A beta[12-28], equipotently impairs post-training memory processing when injected into different limbic system structures.[Pubmed:7874511]
Brain Res. 1994 Nov 14;663(2):271-6.
Previously, amyloid beta-protein (A beta) fragments 1-28, 12-28 and 12-20 were found to impair retention in mice when injected intracerebroventricularly after footshock active avoidance training. We now have measured the dose-dependence of amnestic effects of peptide 12-28 stereotactically injected into amygdala, caudate, hippocampus, mammillary bodies and septum, which limbic structures are known to be involved in memory processing and into the medial thalamus, which largely is involved in sensory processing during training. Peptide 12-28 impaired retention with remarkably similar efficacy when injected into limbic structures but was not at all amnestic upon thalamic injection. Present results together with those in the literature lead us to suggest that A beta may exert dysregulatory cognitive effects by incoordination of K(+)-channel function in neurons, glia and endothelial cells.


