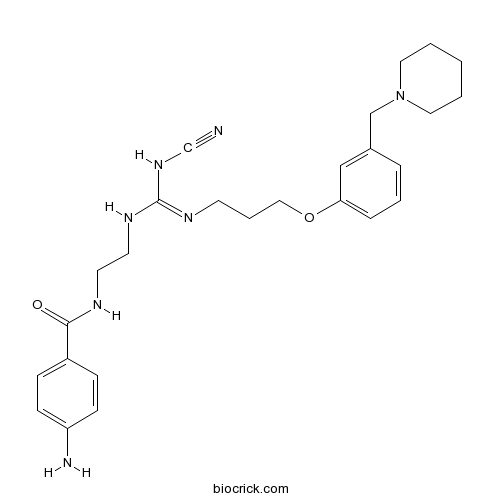
AminopotentidineH2 antagonist CAS# 140873-26-3 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 140873-26-3 | SDF | Download SDF |
| PubChem ID | 164435 | Appearance | Powder |
| Formula | C26H35N7O2 | M.Wt | 477.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| Chemical Name | 4-amino-N-[2-[[N-cyano-N'-[3-[3-(piperidin-1-ylmethyl)phenoxy]propyl]carbamimidoyl]amino]ethyl]benzamide | ||
| SMILES | C1CCN(CC1)CC2=CC(=CC=C2)OCCCN=C(NCCNC(=O)C3=CC=C(C=C3)N)NC#N | ||
| Standard InChIKey | CICDSYWWNDGAGD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H35N7O2/c27-20-32-26(31-14-13-29-25(34)22-8-10-23(28)11-9-22)30-12-5-17-35-24-7-4-6-21(18-24)19-33-15-2-1-3-16-33/h4,6-11,18H,1-3,5,12-17,19,28H2,(H,29,34)(H2,30,31,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | H2 antagonist (KB values are 220 and 280 nM at human and guinea pig H2 receptors respectively) and precursor for the synthesis of the [125I]-iodo derivative. |

Aminopotentidine Dilution Calculator

Aminopotentidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0938 mL | 10.469 mL | 20.938 mL | 41.876 mL | 52.3451 mL |
| 5 mM | 0.4188 mL | 2.0938 mL | 4.1876 mL | 8.3752 mL | 10.469 mL |
| 10 mM | 0.2094 mL | 1.0469 mL | 2.0938 mL | 4.1876 mL | 5.2345 mL |
| 50 mM | 0.0419 mL | 0.2094 mL | 0.4188 mL | 0.8375 mL | 1.0469 mL |
| 100 mM | 0.0209 mL | 0.1047 mL | 0.2094 mL | 0.4188 mL | 0.5235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ICI 215,001 hydrochloride
Catalog No.:BCC5688
CAS No.:140850-02-8
- 1-Hydroxymethyl-beta-carboline glucoside
Catalog No.:BCN7026
CAS No.:1408311-12-5
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- PF 06465469
Catalog No.:BCC6268
CAS No.:1407966-77-1
- Scandine Nb-oxide
Catalog No.:BCN7504
CAS No.:140701-69-5
- 4-Aza-5androstan-1-ene- 3-one-17carboxylic acid
Catalog No.:BCC8693
CAS No.:140700-63-6
- 1-Deoxydihydroceramide-1-sulfonic acid
Catalog No.:BCC4964
CAS No.:1407-03-0
- Levatin
Catalog No.:BCN2531
CAS No.:140670-84-4
- Kadsurenin D
Catalog No.:BCN6603
CAS No.:140669-89-2
- 12-Hydroxyjasmonic acid
Catalog No.:BCN6224
CAS No.:140631-27-2
- 4-Ethylsyringol
Catalog No.:BCN3541
CAS No.:14059-92-8
- Cassipourine
Catalog No.:BCN2154
CAS No.:14051-10-6
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
- (2R,3S)-Chlorpheg
Catalog No.:BCC6805
CAS No.:140924-23-8
- Benzo-18-crown-6 ether
Catalog No.:BCC8850
CAS No.:14098-24-9
- Benzo-15-crown 5-ether
Catalog No.:BCC8849
CAS No.:14098-44-3
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- 2-Aminoethanol
Catalog No.:BCN1756
CAS No.:141-43-5
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
Synthesis and pharmacological characterization of novel fluorescent histamine H2-receptor ligands derived from aminopotentidine.[Pubmed:16730977]
Bioorg Med Chem Lett. 2006 Aug 1;16(15):3886-90.
In an effort to develop a non-radioactive alternative to the [3H]tiotidine and [125I]iodoAminopotentidine binding assays for the histamine H2-receptor (H2R), primary amines related to Aminopotentidine were prepared and coupled with the succinimidyl esters of the bulky fluorescent dyes S0536 and BODIPY 650/665-X. The primary amines exhibited different degrees of antagonistic potency at the human and guinea pig H2R. Surprisingly, one compound (5) coupled to the cyanine dye S0536 acted as potent partial agonist/antagonist at the H2R (KB approximately 50 nM; EC50 approximately 100-150 nM). Compounds coupled to the BODIPY dye exhibited moderately high H2R-affinity, too. Thus, the H2R accommodates bulky fluorophores, probably through interaction with extracellular receptor domains. The compounds presented herein provide a starting point for the optimization of fluorescent H2R ligands with respect to affinity and fluorescence as valuable tools to analyze the molecular mechanisms of H2R activation.
The pharmacological activity of some new fluorescent small molecule histamine H2 receptor (H2-R) ligands, derived from aminopotentidine and squaramide, in the GTPase assay.[Pubmed:20509312]
Rev Med Chir Soc Med Nat Iasi. 2010 Jan-Mar;114(1):255-9.
UNLABELLED: We characterized the pharmacological activity of some new fluorescent and nonfluorescent ligands derived from Aminopotentidine and squaramide with respect to the interaction with human (h) and guinea pig (gp) H2R species isoforms on Spodoptera frugiperda (Sf9) cell membranes expressing H2R-Gsalpha fusion proteins. MATERIAL AND METHOD: The pharmacological studies were performed in a radioactivity-based GTPase assay. Non-linear curve ?tting was performed using the computer program Prism 4.0 (GraphPad-Prism). RESULTS: Interestingly is that the Aminopotentidine ligands labeled with S0536 dye showed a higher activity than the squaramide ligands labeled with the same fluorophor. Moreover, for some cases, the bulky fluorescent groups can even substantially increase ligand affinity. CONCLUSION: The study of comparative antagonist activity of new H2R ligands derived from Aminopotentidine and squaramide in the GTPase assay suggests that there is no common, lock' for all, keys' at the H2R.
Iodoaminopotentidine and related compounds: a new class of ligands with high affinity and selectivity for the histamine H2 receptor.[Pubmed:1613748]
J Med Chem. 1992 Jun 12;35(12):2231-8.
The synthesis and biological evaluation of a new class of histamine H2 antagonists with N-cyano-N'-[omega-[3-(1-piperidinylmethyl)phenoxy] alkyl]guanidine partial structure are described as part of an extensive research program to find model compounds for the development of new radioligands with high H2 affinity and specific activity. High receptor affinity is achieved by an additional (substituted) aromatic ring, which is connected with the third guanidine N by a carbon chain spacer and an amine, carboxamide, ester, or sulfonamide link ("polar group"). In functional studies for H2 antagonistic activity and other pharmacological actions [e.g. H1 antihistaminic, antimuscarinic, antiadrenergic (alpha 1, beta 1), 5-HT2 blocking activity] in the isolated guinea pig atrium and ileum and rat aorta and tail artery, the compounds proved to be highly potent and selective histamine H2 receptor antagonists. The H2 antagonistic activity is mainly depending on the length of both the N'-alkyl chain (chain A) and the N"-spacer (chain B). Compounds with a C3 chain A and a C2 chain B are most potent in the preferred group of substances, i.e., the carboxamide series. A wide variety of substituents at the aromatic ring is tolerated, among them iodine, amino, and azido groups. These compounds are up to 32 times more potent than cimetidine in the isolated guinea pig right atrium. The replacement of the carboxamide by an ester group (44c) is well tolerated, while replacement of the cyanoguanidine by an urea group results in nearly 100-fold decrease in activity (46c,e). The iodinated benzamides are among the most potent H2 antagonists known so far. The [125I]-labeled form of 31f ([125I]iodoAminopotentidine, [125I]-N-[2-(4-amino-3-iodobenzamido) ethyl]-N'-cyano-N"-[3-[3-(1-piperidinylmethyl) phenoxy]propyl]guanidine) and its photolabile analogue 31h ([125I]iodoazidopotentidine, [125I]-N-[2-(4-azido-3- iodobenzamido)ethyl]-N'-cyano-N"-[3-[3-(1-piperidinyl-methyl)pheno xy] propyl]guanidine) proved to be useful probes for reversible and irreversible labeling of the histamine H2 receptor. Radioligand binding studies in guinea pig cerebral membranes revealed considerably higher H2 receptor affinity for 31f (pKi = 9.15), 31h (pKi = 8.58), and some analogues than functional experiments (guinea pig atrium), presumably reflecting an easier access to the H2 receptors in membranes.
Reversible and irreversible labeling and autoradiographic localization of the cerebral histamine H2 receptor using [125I]iodinated probes.[Pubmed:2308927]
Proc Natl Acad Sci U S A. 1990 Mar;87(5):1658-62.
IodoAminopotentidine (I-APT)--i.e., N-[2-(4-amino-3-iodobenzamido)ethyl]-N'-cyano-N''-(3-[3- (1-piperidinylmethyl)phenoxy]propyl)guanidine--represents one of the most potent H2-receptor antagonists known so far. In membranes of guinea pig brain 125I-APT bound reversibly, selectively, and with high affinity (Kd = 0.3 nM) to a homogeneous population of sites unambiguously identified as H2 receptors by inhibition studies conducted with a large panel of antagonists. 125I-APT binding was also inhibited by histamine, and the effect was modulated by a guanyl nucleotide, which is consistent with the association of the H2 receptor with a guanine nucleotide binding regulatory protein. The low nonspecific binding of 125I-APT generated high contrast autoradiographic pictures in brain sections and established the precise distribution of H2 receptors. Their highly heterogeneous distribution and laminated pattern in some areas--e.g., cerebral and hippocampal cortices--suggest their major association with neuronal elements. These localizations were more consistent than those of H1 receptors with the distribution of histaminergic projections, indicating that H2 receptors mediate a larger number of postsynaptic actions of histamine--e.g., in striatum. Colocalizations of H1 and H2 receptors in some areas account for their known synergistic interactions in cAMP formation induced by histamine. The distribution of 125I-APT binding sites did not strictly parallel that of the H2-receptor-linked adenylate cyclase activity, which may reflect heterogeneity among H2 receptors. After UV irradiation and SDS/PAGE analysis, [125I]iodoazidopotentidine (125I-AZPT), a photoaffinity probe derived from 125I-APT, was covalently incorporated in several peptides, among which the labeling of two peptides of 59 and 32 kDa was prevented by H2 antagonists, suggesting that they correspond to H2-receptor binding peptides or proteolysis products of the latter. These probes should be useful for sensitive radioassays, localization, purification, and molecular studies of the H2 receptor, which were previously impracticable.


