Alpha-Angelica lactoneCAS# 591-12-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 591-12-8 | SDF | Download SDF |
| PubChem ID | 11559 | Appearance | Liquid |
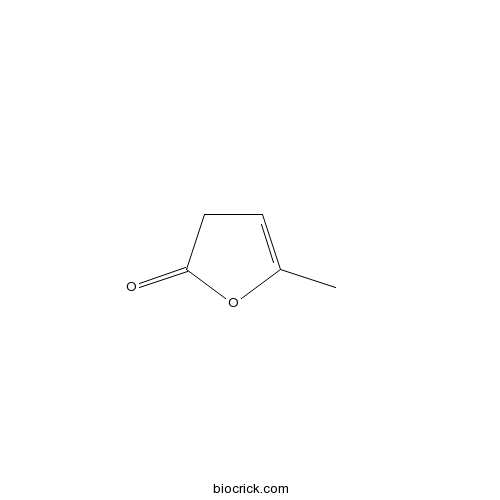
| Formula | C5H6O2 | M.Wt | 98.10 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-methyl-3H-furan-2-one | ||
| SMILES | CC1=CCC(=O)O1 | ||
| Standard InChIKey | QOTQFLOTGBBMEX-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Alpha-Angelica lactone has cardiotonic activity, may exert their effects by providing an increased contraction-dependent calcium pool to be released upon systolic depolarization. Alpha-Angelicalactone can induce increased glutathione (GSH) S-transferase activity in the liver and small intestine in female ICR/Ha mice, it is an inhibitor of chemical carcinogenesis. |
| Targets | Calcium Channel | ATPase |
| In vitro | Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents.[Pubmed: 6278195]J Natl Cancer Inst. 1982 Mar;68(3):493-6.Benzyl isothiocyanate, beta-naphthoflavone, coumarin, Alpha-Angelica lactone, disulfiram, indole-3-carbinol and indole-3-acetonitrile induced increased glutathione (GSH) S-transferase activity in the liver and small intestine in female ICR/Ha mice. |
| Kinase Assay | The influence of ouabain and alpha angelica lactone on calcium metabolism of dog cardiac microsomes.[Pubmed: 4236805]J Clin Invest. 1969 Feb;48(2):229-34.The influence of ouabain and Alpha-Angelica lactone on (45)calcium accumulation in cardiac microsomes was studied. |
| Structure Identification | Org Lett. 2011 Jun 17;13(12):3056-9.Catalytic asymmetric vinylogous Mannich-type (AVM) reaction of nonactivated α-angelica lactone.[Pubmed: 21595425]A direct highly diastereo- and enantioselective asymmetric vinylogous Mannich-type (AVM) reaction of aldimines with nonactivated natural Alpha-Angelica lactone has been successfully developed. It was demonstrated that the nonactivated natural Alpha-Angelica lactone is a useful vinylogous nucleophile to give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives. The N,N'-dioxide L2-Sc(III) complex is efficient toward the obtention of a range of corresponding products with adjacent quaternary and tertiary stereocenters in excellent dr and ee values. |

Alpha-Angelica lactone Dilution Calculator

Alpha-Angelica lactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 10.1937 mL | 50.9684 mL | 101.9368 mL | 203.8736 mL | 254.842 mL |
| 5 mM | 2.0387 mL | 10.1937 mL | 20.3874 mL | 40.7747 mL | 50.9684 mL |
| 10 mM | 1.0194 mL | 5.0968 mL | 10.1937 mL | 20.3874 mL | 25.4842 mL |
| 50 mM | 0.2039 mL | 1.0194 mL | 2.0387 mL | 4.0775 mL | 5.0968 mL |
| 100 mM | 0.1019 mL | 0.5097 mL | 1.0194 mL | 2.0387 mL | 2.5484 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Rhododendrol
Catalog No.:BCN7091
CAS No.:59092-94-3
- Albaspidin AP
Catalog No.:BCN2398
CAS No.:59092-91-0
- Dehydrotoxicarol
Catalog No.:BCN3991
CAS No.:59086-93-0
- Atropine sulfate monohydrate
Catalog No.:BCC3728
CAS No.:5908-99-6
- 8-Hydroxyhyperforin 8,1-hemiacetal
Catalog No.:BCN4091
CAS No.:59014-02-7
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
- Bethanechol chloride
Catalog No.:BCC4566
CAS No.:590-63-6
- Betaine hydrochloride
Catalog No.:BCN6304
CAS No.:590-46-5
- Tolazoline HCl
Catalog No.:BCC4321
CAS No.:59-97-2
- Levodopa
Catalog No.:BCN1098
CAS No.:59-92-7
- Nitrofurazone
Catalog No.:BCC3825
CAS No.:59-87-0
- Nicotinic acid
Catalog No.:BCN8328
CAS No.:59-67-6
- Misoprostol
Catalog No.:BCC5240
CAS No.:59122-46-2
- Neoisoliquiritin
Catalog No.:BCN2936
CAS No.:59122-93-9
- PSB 10 hydrochloride
Catalog No.:BCC7238
CAS No.:591771-91-4
- Sulforaphene
Catalog No.:BCN8179
CAS No.:592-95-0
- beta-Dihydroplumericinic acid
Catalog No.:BCN4092
CAS No.:59204-61-4
- 8(14),15-Isopimaradiene-3,18-diol
Catalog No.:BCN4093
CAS No.:59219-64-6
- Darutoside
Catalog No.:BCN4094
CAS No.:59219-65-7
- Erigeroside
Catalog No.:BCC8171
CAS No.:59219-76-0
- Laurocapram
Catalog No.:BCN8308
CAS No.:59227-89-3
- Chikusetsu Saponin Ib
Catalog No.:BCC8308
CAS No.:59252-87-8
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents.[Pubmed:6278195]
J Natl Cancer Inst. 1982 Mar;68(3):493-6.
Benzyl isothiocyanate, beta-naphthoflavone, coumarin, alpha-angelicalactone, disulfiram, indole-3-carbinol and indole-3-acetonitrile induced increased glutathione (GSH) S-transferase activity in the liver and small intestine in female ICR/Ha mice. All seven compounds are inhibitors of chemical carcinogenesis. In additional work, several dietary constituents increased GSH S-transferase activity. Consumption of diets containing dried powdered preparations of brussels sprouts, cabbage, coffee beans, or tea leaves resulted in increased GSH S-transferase activity. Mice fed an unrefined diet (Purina Rat Chow) had a higher GSH S-transferase activity than those fed a semipurified diet. The results of the present study indicated that the composition of the diet can alter the activity of an important enzyme system having the capacity to detoxify chemical carcinogens.
The influence of ouabain and alpha angelica lactone on calcium metabolism of dog cardiac microsomes.[Pubmed:4236805]
J Clin Invest. 1969 Feb;48(2):229-34.
The influence of ouabain and alpha angelica lactone on (45)calcium accumulation in cardiac microsomes was studied. Calcium binding (accumulation in the absence of excess oxalate or phosphate) was augmented by both ouabain and alpha angelica lactone in the presence of adenosine triphosphate (ATP) but unaffected in its absence. Calcium turnover (defined as the change in (45)Ca(++) bound to the microsomes after the specific activity is changed) was studied to determine if the augmented bound pool was freely exchangeable at equilibrium. Ouabain and alpha angelica lactone augmented calcium turnover in both the presence and absence of ATP. Calcium-stimulated ATPase was increased by both agents.It is proposed that these two unsaturated lactones, with known cardiotonic activity, may exert their effects by providing an increased contraction-dependent calcium pool to be released upon systolic depolarization.
Catalytic asymmetric vinylogous Mannich-type (AVM) reaction of nonactivated alpha-angelica lactone.[Pubmed:21595425]
Org Lett. 2011 Jun 17;13(12):3056-9.
A direct highly diastereo- and enantioselective asymmetric vinylogous Mannich-type (AVM) reaction of aldimines with nonactivated natural Alpha-Angelica lactone has been successfully developed. It was demonstrated that the nonactivated natural Alpha-Angelica lactone is a useful vinylogous nucleophile to give the chiral delta-amino gamma,gamma-disubstituted butenolide carbonyl derivatives. The N,N'-dioxide L2-Sc(III) complex is efficient toward the obtention of a range of corresponding products with adjacent quaternary and tertiary stereocenters in excellent dr and ee values.


