Aliskiren HemifumaratePotent renin inhibitor; antihypertensive CAS# 173334-58-2 |
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 173334-58-2 | SDF | Download SDF |
| PubChem ID | 6918427 | Appearance | Powder |
| Formula | C64H110N6O16 | M.Wt | 1219.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SPP 100 | ||
| Solubility | H2O : ≥ 50 mg/mL (41.00 mM) *"≥" means soluble, but saturation unknown. | ||
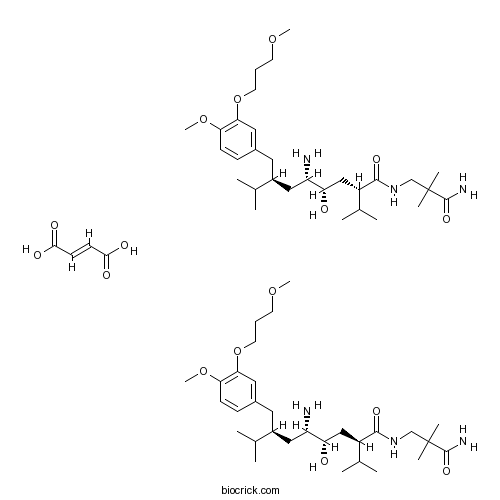
| Chemical Name | (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2-ylnonanamide;(E)-but-2-enedioic acid | ||
| SMILES | CC(C)C(CC1=CC(=C(C=C1)OC)OCCCOC)CC(C(CC(C(C)C)C(=O)NCC(C)(C)C(=O)N)O)N.CC(C)C(CC1=CC(=C(C=C1)OC)OCCCOC)CC(C(CC(C(C)C)C(=O)NCC(C)(C)C(=O)N)O)N.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | KLRSDBSKUSSCGU-KRQUFFFQSA-N | ||
| Standard InChI | InChI=1S/2C30H53N3O6.C4H4O4/c2*1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36;5-3(6)1-2-4(7)8/h2*10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35);1-2H,(H,5,6)(H,7,8)/b;;2-1+/t2*22-,23-,24-,25-;/m00./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent renin inhibitor (IC50 = 0.6 and 80 nM for human and rat respectively). Exhibits selectivity for renin over a range of other aspartic proteinases (>5000 nM). Lowers blood pressure in a hypertensive rodent model. Orally active. |

Aliskiren Hemifumarate Dilution Calculator

Aliskiren Hemifumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8199 mL | 4.0997 mL | 8.1995 mL | 16.399 mL | 20.4987 mL |
| 5 mM | 0.164 mL | 0.8199 mL | 1.6399 mL | 3.2798 mL | 4.0997 mL |
| 10 mM | 0.082 mL | 0.41 mL | 0.8199 mL | 1.6399 mL | 2.0499 mL |
| 50 mM | 0.0164 mL | 0.082 mL | 0.164 mL | 0.328 mL | 0.41 mL |
| 100 mM | 0.0082 mL | 0.041 mL | 0.082 mL | 0.164 mL | 0.205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aliskiren (CGP 60536) is the first in a class of drugs called direct renin inhibitors.Aliskiren current licensed indication is essential (primary) hypertension.
- Aliskiren
Catalog No.:BCC1338
CAS No.:173334-57-1
- Isorhamnetin 3-glucoside-7-rhamnoside
Catalog No.:BCN1528
CAS No.:17331-71-4
- Garciniaxanthone E
Catalog No.:BCN1114
CAS No.:173294-74-1
- TC-E 5003
Catalog No.:BCC8008
CAS No.:17328-16-4
- 2,5-Dihydroxy-1-methoxyxanthone
Catalog No.:BCN7577
CAS No.:173220-32-1
- Broussonetine A
Catalog No.:BCN2515
CAS No.:173220-07-0
- Clomipramine HCl
Catalog No.:BCC5036
CAS No.:17321-77-6
- 4-Beta-Hydroxycholesterol
Catalog No.:BCN2752
CAS No.:17320-10-4
- SQ 22536
Catalog No.:BCC7065
CAS No.:17318-31-9
- Centaureidin
Catalog No.:BCN2575
CAS No.:17313-52-9
- Ganoderic acid DM
Catalog No.:BCN1113
CAS No.:173075-45-1
- Rhoifolin
Catalog No.:BCN1112
CAS No.:17306-46-6
- Pelargonidin-3,5-O-diglucoside chloride
Catalog No.:BCN1527
CAS No.:17334-58-6
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
- Ficusin A
Catalog No.:BCN1115
CAS No.:173429-83-9
- HMN-214
Catalog No.:BCC2517
CAS No.:173529-46-9
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Compound W
Catalog No.:BCC2341
CAS No.:173550-33-9
- 3,5-Dinitro-Tyr-OH
Catalog No.:BCC3331
CAS No.:17360-11-1
- Oxotremorine sesquifumarate
Catalog No.:BCC6814
CAS No.:17360-35-9
- 2,3-Didehydrosomnifericin
Catalog No.:BCN8005
CAS No.:173614-88-5
- Desoxygambogenin
Catalog No.:BCN3068
CAS No.:173614-93-2
- 3-Galloylquinic acid
Catalog No.:BCN3732
CAS No.:17365-11-6
Simultaneous Determination of Aliskiren Hemifumarate, Amlodipine Besylate and Hydrochlorothiazide in Spiked Human Plasma Using UPLC-MS/MS.[Pubmed:25575509]
J Chromatogr Sci. 2015 Aug;53(7):1178-84.
A sensitive UPLC-MS/MS method was developed and validated for simultaneous estimation of Aliskiren Hemifumarate (ALS), amlodipine besylate (AML) and hydrochlorothiazide (HCZ) in spiked human plasma using valsartan as an internal standard (IS). Liquid-liquid extraction was used for purification and pre-concentration of analytes. The mobile phase consisted of 0.1% formic acid in ammonium acetate buffer (0.02 M, pH 3.5) and methanol (25:75, v/v), flowing through XBridge BEH (50 x 2.1 mm ID, 5 microm) C18 column, at a flow rate of 0.6 mL min(-1). Multiple reaction monitoring (MRM) transitions were measured using an electrospray source in the positive ion mode for ALS and AML, whereas HCZ and IS were measured in negative ion mode. Validation of the method was performed as per US-FDA guidelines with linearity in the range of 2.0-400.0, 0.3-25.0 and 5.0-400.0 ng mL(-1) for ALS, AML and HCZ, respectively. In human plasma, ALS, AML and HCZ were stable for at least 1 month at -70 +/- 5 degrees C and for at least 6 h at ambient temperature. After extraction from plasma, the reconstituted samples of ALS, AML and HCZ were stable in the autosampler at ambient temperature for 6 h. The LC-MS/MS method is suitable for bioequivalence and pharmacokinetic studies of this combination.
A validated LC method for the determination of the enantiomeric purity of aliskiren hemifumarate in bulk drug samples.[Pubmed:22732253]
J Chromatogr Sci. 2012 Oct;50(9):799-802.
High-performance liquid chromatography enantioseparation of Aliskiren Hemifumarate was accomplished on an immobilized-type Chiralpak IC chiral stationary phase under both polar organic and reversed-phase modes. A simple analytical method was developed and validated using a mixture of acetonitrile-n-butylamine 100:0.1 (v/v/) as a mobile phase with a flow rate maintained at 1.0 mL/min. Ultraviolet detection was carried out at 228 nm. Resolution between the two enantiomers was greater than 3.0. This method was capable of detecting the R-isomer to a level of 0.2 mug/mL. The method was validated as per International Conference on Harmonization guidelines and found to be robust. The method is very useful for routine evaluation of the quality of Aliskiren Hemifumarate in bulk drug manufacturing units.
Steady-state and synchronous spectrofluorimetric methods for simultaneous determination of aliskiren hemifumarate and amlodipine besylate in dosage forms.[Pubmed:24687516]
Luminescence. 2014 Nov;29(7):878-83.
Aliskiren Hemifumarate (ALS) and amlodipine besylate (AML) were simultaneously determined by two different spectrofluorimetric techniques. The first technique depends on direct measurement of the steady-state fluorescence intensities of ALS and AML at 313 nm and 452 nm upon excitation at 290 and 375 nm, respectively, in a solvent composed of methanol and water (10: 90, v/v). The second technique utilizes synchronous fluorimetric quantitative screening of the emission spectra of ALS and AML at 272 and 366 nm, respectively using Deltalambda of 97 nm. Effects of different solvents and surfactants on relative fluorescence intensity were studied. The method was validated according to ICH guidelines. Linearity, accuracy and precision were found to be satisfactory in both techniques over the concentration ranges of 1-15 and 0.4-4 microg/mL for ALS and AML, respectively. In the first technique, limit of detection and limit of quantification were estimated and found to be 0.256 and 0.776 microg/mL for ALS as well as 0.067 and 0.204 microg/mL for AML, respectively. Also, limit of detection and limit of quantification were calculated in the synchronous method and found to be 0.293 and 0.887 microg/mL for ALS as well as 0.034 and 0.103 microg/mL for AML, respectively. The methods were successfully applied for the determination of the two drugs in their co-formulated tablets. The results were compared statistically with reference methods and no significant difference was found. The developed methods are rapid, sensitive, inexpensive and accurate for the quality control and routine analysis of the cited drugs in bulk and in pharmaceutical preparations without pre-separation.
Simultaneous determination of aliskiren hemifumarate, amlodipine besylate, and hydrochlorothiazide in their triple mixture dosage form by capillary zone electrophoresis.[Pubmed:24574149]
J Sep Sci. 2014 May;37(9-10):1206-13.
A novel, specific, reliable, and accurate capillary zone electrophoretic method was developed and validated for the simultaneous determination of Aliskiren Hemifumarate, amlodipine besylate, and hydrochlorothiazide in their triple mixture dosage form. Separation was carried out in a fused-silica capillary (57.0 cm total length and 50.0 cm effective length, 75.6 mum internal diameter) by applying a potential of 17 kV and a running buffer consisting of 40 mM phosphate buffer at pH 6.0 with UV detection at 245 nm. The method was suitably validated with respect to specificity, linearity, LOD, and LOQ, accuracy, precision, and robustness. The method showed good linearity in the ranges 1-10, 2.5-25, and 30-300 mug/mL with LODs of 0.11, 0.33, and 5.83 mug/mL for amlodipine besylate, hydrochlorothiazide, and Aliskiren Hemifumarate, respectively. The proposed method was successfully applied for the analysis of the studied drugs in their coformulated tablets. The results of the proposed method were statistically compared with those obtained by the RP-HPLC reference method revealing no significant differences in the performance of the methods regarding accuracy and precision.
Drug discovery for heart failure: a new era or the end of the pipeline?[Pubmed:17268484]
Nat Rev Drug Discov. 2007 Feb;6(2):127-39.
Although there have been significant advances in the therapy of heart failure in recent decades, such as the introduction of beta-blockers and antagonists of the renin-angiotensin system, there is still a major unmet need for better therapies for many patients with heart failure. However, disappointment related to late-stage clinical failures of a number of novel agents, including endothelin antagonists and tumour-necrosis factor blockers, has reduced the impetus of drug development in this field. Here, we review possible targets for heart failure therapy that have emerged from recent progress in our understanding of the underlying disease mechanisms, and highlight key issues that need to be addressed to improve the chances of success of novel therapies directed against these targets.
Aliskiren: the first renin inhibitor for clinical treatment.[Pubmed:18340340]
Nat Rev Drug Discov. 2008 May;7(5):399-410.
The first evidence of the existence of renin was presented over 100 years ago. However, the importance of renin and the renin-angiotensin system in the pathogenesis of cardiovascular disease was only fully realized in the 1970s. It was another 20 years before the first inhibitors of renin were available for clinical research. Here, we describe the discovery and development of aliskiren, an orally active renin inhibitor, which became the first drug in its class to receive regulatory approval. In 2007, it was approved for the treatment of hypertension by the US Food and Drug Administration and the European Medicines Agency.


