Acetyl-StrophanthidinCAS# 60-38-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60-38-8 | SDF | Download SDF |
| PubChem ID | 6062 | Appearance | Powder |
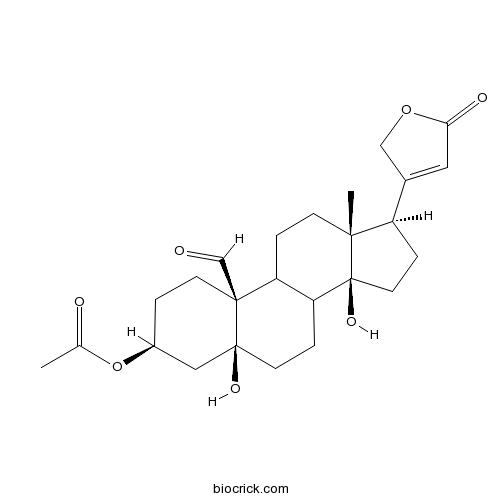
| Formula | C25H34O7 | M.Wt | 446.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3S,5S,10S,13R,14S,17R)-10-formyl-5,14-dihydroxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate | ||
| SMILES | CC(=O)OC1CCC2(C3CCC4(C(CCC4(C3CCC2(C1)O)O)C5=CC(=O)OC5)C)C=O | ||
| Standard InChIKey | JLZAERUVCODZQO-JJTVRZIESA-N | ||
| Standard InChI | InChI=1S/C25H34O7/c1-15(27)32-17-3-8-23(14-26)19-4-7-22(2)18(16-11-21(28)31-13-16)6-10-25(22,30)20(19)5-9-24(23,29)12-17/h11,14,17-20,29-30H,3-10,12-13H2,1-2H3/t17-,18+,19?,20?,22+,23-,24-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Acetyl-Strophanthidin Dilution Calculator

Acetyl-Strophanthidin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2396 mL | 11.1982 mL | 22.3964 mL | 44.7928 mL | 55.991 mL |
| 5 mM | 0.4479 mL | 2.2396 mL | 4.4793 mL | 8.9586 mL | 11.1982 mL |
| 10 mM | 0.224 mL | 1.1198 mL | 2.2396 mL | 4.4793 mL | 5.5991 mL |
| 50 mM | 0.0448 mL | 0.224 mL | 0.4479 mL | 0.8959 mL | 1.1198 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.224 mL | 0.4479 mL | 0.5599 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetamide
Catalog No.:BCN4114
CAS No.:60-35-5
- Linoleic acid
Catalog No.:BCN3821
CAS No.:60-33-3
- (6-)ε-Aminocaproic acid
Catalog No.:BCC4888
CAS No.:60-32-2
- Acetylcholine chloride
Catalog No.:BCN2197
CAS No.:60-31-1
- H-Tyr-OH
Catalog No.:BCC3123
CAS No.:60-18-4
- Guanethidine Sulfate
Catalog No.:BCC3789
CAS No.:60-02-6
- EDTA
Catalog No.:BCC7493
CAS No.:60-00-4
- Tagitinin A
Catalog No.:BCN4102
CAS No.:59979-61-2
- Tagitinin F
Catalog No.:BCN4101
CAS No.:59979-57-6
- Carlinoside
Catalog No.:BCN2853
CAS No.:59952-97-5
- Malotilate
Catalog No.:BCC1196
CAS No.:59937-28-9
- 3',4'-dihydro-3'-hydroxy-Xanthyletin
Catalog No.:BCN3680
CAS No.:5993-18-0
- Tetracycline
Catalog No.:BCC9176
CAS No.:60-54-8
- Methimazole
Catalog No.:BCC3812
CAS No.:60-56-0
- Veratramine
Catalog No.:BCN2965
CAS No.:60-70-8
- Antipyrine
Catalog No.:BCC8834
CAS No.:60-80-0
- Phlorizin
Catalog No.:BCN4126
CAS No.:60-81-1
- Phloretin
Catalog No.:BCN4128
CAS No.:60-82-2
- Adenosine cyclophosphate
Catalog No.:BCN2190
CAS No.:60-92-4
- N-Me-DL-Ala-OH.HCl
Catalog No.:BCC2618
CAS No.:600-21-5
- 11Beta-hydroxyprogesterone
Catalog No.:BCN2211
CAS No.:600-57-7
- Oblongine
Catalog No.:BCN4103
CAS No.:60008-01-7
- Glabrene
Catalog No.:BCN6692
CAS No.:60008-03-9
- Chlormethiazole hydrochloride
Catalog No.:BCC6830
CAS No.:6001-74-7
Evidence for a sodium-dependent potassium conductance in frog myelinated axon.[Pubmed:7477959]
Neuroscience. 1995 Sep;68(2):487-95.
After blockade of the voltage-dependent potassium conductances by intracellular application of 4-aminopyridine and tetraethylammonium in frog myelinated axons, a set of brief (0.1 ms) intracellular depolarizing pulses or a long (200 ms) depolarizing pulse evoked a train of action potentials. Under both experimental conditions a hyperpolarizing afterpotential appeared (duration 367 ms +/- 34, mean +/- S.E., n = 15). The purpose of this study was to investigate the properties of this hyperpolarizing afterpotential. It was found that the hyperpolarizing afterpotential increases in amplitude with: (1) the number of sodium-dependent action potentials; (2) action potential broadening (following potassium channels blockade); and (3) the level of depolarization during a current step. Application of tetrodotoxin prevented the activation of the hyperpolarizing afterpotential by any of the above stimuli. The hyperpolarizing afterpotential was unaffected by: (1) 8-Acetyl-Strophanthidin, an agent that poisons the electrogenic pumping in the axon; (2) blocking calcium influx with extracellular 10 mM magnesium or 2 mM manganese; and (3) buffering of the intracellular calcium, using EGTA in the recording microelectrode. Extracellular application of tetraethylammonium, but not 4-aminopyridine, reduced the hyperpolarizing afterpotential. The hyperpolarizing afterpotential reversed at >> -92 mV. Increasing the external potassium concentration from 2 to 10 mM shifted the reversal potential +14.5 mV, indicating that the hyperpolarizing afterpotential is a potassium mediated conductance.(ABSTRACT TRUNCATED AT 250 WORDS)
Assessment of the hemodynamic response to acetyl-strophanthidin by Doppler echocardiography in normal subjects and in those with coronary artery disease or idiopathic dilated cardiomyopathy.[Pubmed:2316465]
Am J Cardiol. 1990 Mar 15;65(11):804-7.
The effect of Acetyl-Strophanthidin, a rapidly acting digitalis-like drug, was measured on peak flow velocity, stroke distance (an index of stroke volume) and minute distance (an index of cardiac output), determined by Doppler echocardiography in 21 subjects with a wide range of left ventricular ejection fractions (12 to 89%, average 47%). For the total study group, peak flow velocity increased from 99 +/- 10 to 110 +/- 13 cm/s (p less than 0.01), and stroke distance increased from 15.1 +/- 3.2 to 16.8 +/- 3.1 cm (p less than 0.01). Minute distance remained unchanged: 1,093 +/- 168 cm before and 1,129 +/- 187 cm after Acetyl-Strophanthidin (difference not significant). Improvement in Doppler parameters of forward blood flow was significantly (p less than 0.001) greater in subjects with left ventricular ejection fractions less than 60% (+17% for peak flow velocity, +22% for stroke distance and +15% for minute distance) than those with left ventricular ejection fractions greater than or equal to 60% (+4% for peak flow velocity, +2% for stroke distance and -8% for minute distance). These data suggest that Doppler echocardiography is a useful method to assess the efficacy of acute digitalis administration in improving forward blood flow.
Effects of acetyl-strophanthidin on left ventricular function and ventricular arrhythmias.[Pubmed:6720517]
Am Heart J. 1984 May;107(5 Pt 1):882-7.
Digitalis drugs can suppress ventricular arrhythmias. It is uncertain whether this effect results from improved left ventricular (LV) function. We utilized radionuclide scanning techniques to evaluate changes in LV ejection fraction (EF) after an infusion of Acetyl-Strophanthidin in 43 patients with frequent ventricular premature beats (VPBs) (44 to 2400/hr). Acetyl-Strophanthidin suppressed ventricular arrhythmia in 17 patients, but LVEF increased in only six of these patients (57% to 67%), while it was unaltered in 11 patients (28% to 30%). In 26 patients ventricular arrhythmia was not suppressed. Fifteen of these patients had an increase in LVEF (60% vs 71%), while this was unchanged in 11 patients (27% vs 29%). Thus no correlation was observed between the positive inotropic and antiarrhythmic action of Acetyl-Strophanthidin on ventricular arrhythmia and LVEF. We conclude that the suppression of VPBs by Acetyl-Strophanthidin is independent of the drug's effects on LV function. Evidence is reviewed suggesting that the antiarrhythmic effect of Acetyl-Strophanthidin on ventricular ectopic activity is due to its vagotonic action.
Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH.[Pubmed:7099919]
Pflugers Arch. 1982 Apr;393(2):171-8.
Intracellular Ca, Na and H selective microelectrodes were used to study the effects of reduction of the extracellular Na concentration, [Na]0, on the free intracellular Ca concentration, [Ca]i, Na activity, (aiNa), and intracellular pH (pHi) in sheep heart Purkinje fibres. 1. Reduction of [Na]0 from 140 mM to 14 mM produced a decrease of aiNa, and increase of free [Ca]i, and normally an increase of resting tension. 2. Inhibition of the Na-K pump by 10(-5) M Acetyl-Strophanthidin produced a slow rise of [Ca]i and resting tension. 3. The magnitude of the increase of free [Ca]i (and tension) produced by [Na]0 reduction was greatly enhanced when the Na-K pump is inhibited by either acetylstrophanthidin or K-free solutions. 4. We suggest that this enhanced rise of free [Ca]i in the presence of Na-K pump inhibition is due to Ca loading of intracellular Ca buffering systems during the pump inhibition. 5. Addition of NH4Cl produced a transient decrease of free [Ca]i that accompanied an alkaline change in pHi. Removal of NH4Cl (which produced a transient intracellular acidification) produced a transient increase of free [Ca]i. We conclude that a close relationship exists between the control of free [Ca]i and pHi which may be due to competition at, or common use of, intracellular buffering systems.


