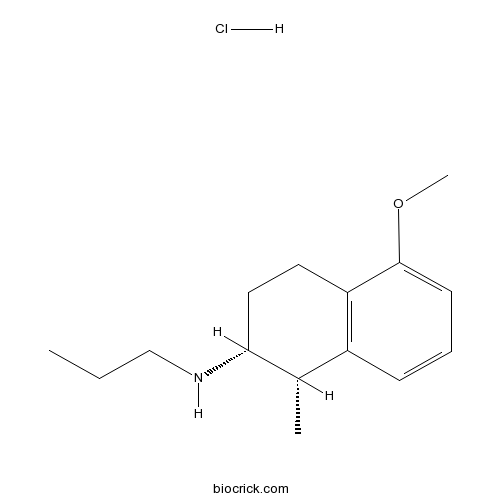
(+)-AJ 76 hydrochlorideDopamine receptor antagonist CAS# 85378-82-1 |
- AVL-292 benzenesulfonate
Catalog No.:BCC1386
CAS No.:1360053-81-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85378-82-1 | SDF | Download SDF |
| PubChem ID | 13755572 | Appearance | Powder |
| Formula | C15H24ClNO | M.Wt | 269.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | (1S,2R)-5-methoxy-1-methyl-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine;hydrochloride | ||
| SMILES | CCCNC1CCC2=C(C1C)C=CC=C2OC.Cl | ||
| Standard InChIKey | KIRYNZFMOLYYQB-YECZQDJWSA-N | ||
| Standard InChI | InChI=1S/C15H23NO.ClH/c1-4-10-16-14-9-8-13-12(11(14)2)6-5-7-15(13)17-3;/h5-7,11,14,16H,4,8-10H2,1-3H3;1H/t11-,14+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine receptor antagonist with preferential action at presynaptic receptors (pKi values are 6.95, 6.67, 6.37, 6.21 and 6.07 at hD3. hD4, hD2S, hD2L and rD2 receptors respectively). |

(+)-AJ 76 hydrochloride Dilution Calculator

(+)-AJ 76 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7063 mL | 18.5316 mL | 37.0631 mL | 74.1262 mL | 92.6578 mL |
| 5 mM | 0.7413 mL | 3.7063 mL | 7.4126 mL | 14.8252 mL | 18.5316 mL |
| 10 mM | 0.3706 mL | 1.8532 mL | 3.7063 mL | 7.4126 mL | 9.2658 mL |
| 50 mM | 0.0741 mL | 0.3706 mL | 0.7413 mL | 1.4825 mL | 1.8532 mL |
| 100 mM | 0.0371 mL | 0.1853 mL | 0.3706 mL | 0.7413 mL | 0.9266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(+)-AJ 76 hydrochloride is an antagonist of dopamine autoreceptor with pKi values of 6.95, 6.67, 6.37, 6.21 and 6.07 for hD3, hD4, hD2S, hD2L and rD2 receptors, respectively.
Dopamine receptor is a G protein-coupled receptor and mainly exists in the vertebrate central nervous system (CNS). Dopamine receptor is a receptor for dopamine and plays a critical role in memory, learning, pleasure, cognition, motivation and fine motor control.
(+)-AJ 76 hydrochloride is a dopamine receptor antagonist. In rats, AJ76 stimulated locomotor activity and increased the levels of 3,4-dihydroxyphenylacetic acid (DOPAC) and HVA in brain, which were dopamine metabolites [1]. In rats injected with cocaine, (+)-A J76 increased the locomotor stimulation during the first 30 min. However, (+)-AJ76 inhibited the later more intense locomotor stimulation and cocaine-induced stereotypies [2]. In vivo, (+)-AJ76 induced dopamine release mainly through interaction with dopamine receptors in the terminal regions of the A9 and A10 dopaminergic fibers. However, (+)-AJ76 increased the level of DOPAC via the somatodendritic autoreceptors [3].
References:
[1]. Kullingsjö H, Carlsson A, Svensson K. Effects of repeated administration of the preferential dopamine autoreceptor antagonist, (+)-AJ76, on locomotor activity and brain DA metabolism in the rat. Eur J Pharmacol, 1991, 205(3): 241-246.
[2]. Piercey MF, Lum JT, Hoffmann WE, et al. Antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-AJ76 and (+)-UH232. Brain Res, 1992; 588(2): 217-222.
[3]. Waters N, Hansson L, Löfberg L, et al. Intracerebral infusion of (+)-AJ76 and (+)-UH232: effects on dopamine release and metabolism in vivo. Eur J Pharmacol, 1994, 251(2-3): 181-190.
- SKF 89976A hydrochloride
Catalog No.:BCC6930
CAS No.:85375-15-1
- 5,19-Epoxy-19R,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1328
CAS No.:85372-72-1
- 5,19-Epoxy-19S,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1329
CAS No.:85372-70-9
- SecinH3
Catalog No.:BCC7503
CAS No.:853625-60-2
- K03861
Catalog No.:BCC6537
CAS No.:853299-07-7
- Trichorabdal A
Catalog No.:BCN4404
CAS No.:85329-59-5
- Hythiemoside A
Catalog No.:BCN4403
CAS No.:853267-91-1
- Ajugalide D
Catalog No.:BCN3665
CAS No.:853247-65-1
- Ajugalide C
Catalog No.:BCN8015
CAS No.:853247-64-0
- PG 01
Catalog No.:BCC7820
CAS No.:853138-65-5
- Anthraquinone-1,5-disulfonic acid disodium salt
Catalog No.:BCC8833
CAS No.:853-35-0
- Dehydroepiandrosterone acetate
Catalog No.:BCC8929
CAS No.:853-23-6
- NVP-BAG956
Catalog No.:BCC1813
CAS No.:853910-02-8
- OGT 2115
Catalog No.:BCC7458
CAS No.:853929-59-6
- Dasatinib hydrochloride
Catalog No.:BCC1517
CAS No.:854001-07-3
- Norcaesalpinin E
Catalog No.:BCN7006
CAS No.:854038-96-3
- (-)-Haplomyrfolin
Catalog No.:BCN3225
CAS No.:85404-48-4
- Ropivacaine mesylate
Catalog No.:BCC9137
CAS No.:854056-07-8
- Rilmenidine Phosphate
Catalog No.:BCC5637
CAS No.:85409-38-7
- S- (+)-Rolipram
Catalog No.:BCC2303
CAS No.:85416-73-5
- (R)-(-)-Rolipram
Catalog No.:BCC5429
CAS No.:85416-75-7
- Caffeic anhydride
Catalog No.:BCN3295
CAS No.:854237-32-4
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
- 4-Chlorotestosterone acetate
Catalog No.:BCC8705
CAS No.:855-19-6
The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats.[Pubmed:14985929]
Psychopharmacology (Berl). 2004 Jul;174(3):341-57.
BACKGROUND: The role of dopamine D(3)/D(2) receptors in the control of locomotion is poorly understood. OBJECTIVES: To examine the influence of selective antagonists at D(3) or D(2) receptors on locomotion in rats, alone and in interaction with the preferential D(3) versus D(2) receptor agonist, PD128,907. METHODS: Affinities of ligands at rat D(2) and cloned, human hD(3), hD(2S), hD(2L) and hD(4) sites were determined by standard procedures. Locomotion was monitored automatically in rats pre-habituated for 30 min to an open-field environment. Extracellular levels of dopamine (DA) were determined by dialysis in the nucleus accumbens and striatum. Drugs were given acutely via the systemic route. RESULTS: PD128,907, which preferentially recognised D(3) versus D(2) sites, biphasically reduced and enhanced locomotion at "low" (0.01-0.63 mg/kg) and "high" (2.5-10 mg/kg) doses, respectively. L741,626 and S23199, which behaved as preferential D(2) versus D(3) receptor antagonists, enhanced the reduction in locomotion evoked by the low dose of PD128,907, blocked the increase provoked by the high dose and suppressed spontaneous locomotion alone. Analogous findings were obtained with haloperidol and raclopride which showed equilibrated affinity at D(2) and D(3) receptors. UH232 and AJ76, which showed a mild preference for D(3) versus D(2) sites, did not modify the effect of a low dose of PD128,907, slightly enhanced the hyperlocomotion elicited by the high dose and exerted little influence on locomotion alone. S14297 and U99194, which acted as preferential D(3) versus D(2) receptor antagonists, abolished the reduction in locomotion elicited by a low dose of PD128,907, potentiated the induction of locomotion by a high dose, and failed to influence locomotion alone. The actions of S14297 were stereoselective inasmuch as they were mimicked by the racemic form, S11566, but not by the inactive enantiomer, S17777. In contrast to S14297, S11566 and U99194, however, S33084, SB269,652, GR218,231 and N-[-4-['-(1-naphtyl)piperazine-1-yl]butyl] anthracene-2-carboxamide ("NGB-1"), highly selective D(3) versus D(2) receptor antagonists, were inactive under all conditions. PD128,907 (0.01-10.0 mg/kg) suppressed dialysate levels of DA in the nucleus accumbens and striatum, actions blocked by L741,626 and haloperidol, yet unaffected by S14297 and S33084. CONCLUSIONS: The facilitatory influence of a "high" dose of PD128,907 upon locomotion is mediated by postsynaptic D(2) receptors and, possibly, countered by their D(3) counterparts. Correspondingly, selective blockade of D(2) but not of D(3) receptors alone suppresses motor function. The reduction in locomotion provoked by a "low" dose of PD128,907 may be mediated by D(2) autoreceptors, but a role of postsynaptic D(3) receptors cannot be excluded. Finally, mechanisms underlying the contrasting influence of chemically diverse D(3) receptor antagonists upon locomotion remain to be elucidated.
Intracerebral infusion of (+)-AJ76 and (+)-UH232: effects on dopamine release and metabolism in vivo.[Pubmed:8149975]
Eur J Pharmacol. 1994 Jan 14;251(2-3):181-90.
The dopamine D3- and autoreceptor preferring antagonists (+)-AJ76 and (+)-UH232 were administered locally in the striatum and the nucleus accumbens. Their effects on dialysate dopamine and 3,4-di-hydroxyphenylacetic acid (DOPAC) were measured and compared with the effects of raclopride. (+)-AJ76 and (+)-UH232 but not raclopride seem to interact primarily with dopamine receptors in the terminal regions of the A9 and A10 dopaminergic fibers to exert their maximal effect on dopamine release in vivo. Thus, (+)-AJ76 and (+)-UH232 seem to recruit different dopamine receptor populations as compared to raclopride. Though the dopamine receptor antagonist-induced effects on dopamine release seem to be mediated mainly by dopamine receptors in the terminal areas, the effects on DOPAC by the different antagonists seem to be mediated mainly via effects elsewhere, presumably at the somatodendritic autoreceptors. Thus, it is suggested that the regulation of extracellular dopamine and DOPAC after treatment with dopamine receptor antagonists are subjected to different control mechanisms.
Effects of repeated administration of the preferential dopamine autoreceptor antagonist, (+)-AJ76, on locomotor activity and brain DA metabolism in the rat.[Pubmed:1817961]
Eur J Pharmacol. 1991 Dec 3;205(3):241-6.
AJ76, the cis-(+)-(1S,2R) enantiomer of 5-methoxy-1-methyl-2-(n-propyl-amino) tetralin is a dopamine autoreceptor antagonist which has shown locomotor stimulatory properties, especially in habituated rats. AJ76 was given repeatedly to male rats at different time intervals and different doses to investigate if tachyphylaxis/tolerance would develop. Tolerance did not occur if AJ76 (300 mumol/kg p.o.) was administered once daily for 7 days, (regarding both stimulation of locomotor activity and increase in brain DOPAC levels). Tolerance occurred after a single dose of 52 mumol/kg s.c. given in the morning followed by the same challenge dose 4 but not 24 h later. When the first dose was decreased to 13 mumol/kg s.c. no tachyphylaxis could be demonstrated regarding stimulation of locomotor activity. It is concluded that AJ76 induces a dose-dependent and short lasting tachyphylaxis, while no tolerance is observed after one week repeated administration. The possible mechanism behind these effects are discussed.
Resolved cis- and trans-2-amino-5-methoxy-1-methyltetralins: central dopamine receptor agonists and antagonists.[Pubmed:3560156]
J Med Chem. 1987 Apr;30(4):602-11.
A series of 35 stereochemically well-defined C1-methyl-substituted derivatives of the potent dopamine (DA) receptor agonist 5-hydroxy-2-(di-n-propylamino)tetralin (5-OH-DPAT) have been synthesized. The compounds were tested for central DA receptor agonistic and antagonistic activity, by use of biochemical and behavioral tests in rats. In addition, the compounds were tested for in vivo interactions with 5,6-dihydroxy-2-(di-n-propylamino)tetralin (DiPr-5,6-ADTN). On the basis of pharmacological activity profiles, the active compounds have been classified into four groups: classical pre- and postsynaptic DA receptor agonists, DA receptor agonists with preferential action at presynaptic receptors, pre- and postsynaptic DA receptor antagonists, and DA receptor antagonists with preferential action at presynaptic receptors. Results obtained indicate that both 2R and 2S enantiomers of C5-oxygenated 2-aminotetralins may be able to bind to DA receptors but that only 2S antipodes are able to activate the receptors. O-Methylation of the C5-oxygenated (1S,2R)-2-amino-1-methyltetralin derivatives tends to increase their DA receptor antagonistic activity, whereas decrease of the size of the N-substituent(s) from n-propyl to ethyl or methyl appears to increase their activity at postsynaptic DA receptors.


