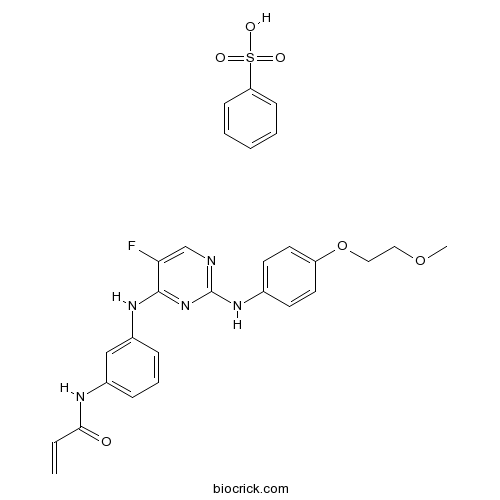
AVL-292 benzenesulfonateSelective Btk inhibitor CAS# 1360053-81-1 |
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1360053-81-1 | SDF | Download SDF |
| PubChem ID | 74892828 | Appearance | Powder |
| Formula | C28H28FN5O6S | M.Wt | 581.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Spebrutinib besylate; CC-292 besylate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | benzenesulfonic acid;N-[3-[[5-fluoro-2-[4-(2-methoxyethoxy)anilino]pyrimidin-4-yl]amino]phenyl]prop-2-enamide | ||
| SMILES | COCCOC1=CC=C(C=C1)NC2=NC=C(C(=N2)NC3=CC(=CC=C3)NC(=O)C=C)F.C1=CC=C(C=C1)S(=O)(=O)O | ||
| Standard InChIKey | ABSXPNGWJFAPRT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22FN5O3.C6H6O3S/c1-3-20(29)25-16-5-4-6-17(13-16)26-21-19(23)14-24-22(28-21)27-15-7-9-18(10-8-15)31-12-11-30-2;7-10(8,9)6-4-2-1-3-5-6/h3-10,13-14H,1,11-12H2,2H3,(H,25,29)(H2,24,26,27,28);1-5H,(H,7,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AVL-292 benzenesulfonate is a potent inhibitor of Btk kinase activity (IC50<0.5 nM, Kinact/Ki=7.69×104 M-1s-1s) in biochemical assays.In Vitro:AVL-292 (CC-292) is a covalent, highly selective, orally active inhibitor of Btk with IC50 value of 0.5 nM. AVL-292 also less potently inhibits Yes, c-Src, Brk, Lyn, and Fyn with IC50s of 723 nM, 1.729 μM, 2.43 μM, 4.4 μM, and 7.15 μM, rspectively. Extensive analysis has revealed that the EC50 of Btk occupancy from a AVL-292 dose-response in Ramos cells (EC50=6 nM) correlated directly with the cellular EC50 of Btk kinase inhibition with AVL-292 (EC50=8 nM). Furthermore, the concentration at which AVL-292 inhibits 90% of Btk activity in Ramos cells is 35 nM while the concentration of AVL-292 required for 90% occupancy of Btk is 39 nM[1]. References: | |||||

AVL-292 benzenesulfonate Dilution Calculator

AVL-292 benzenesulfonate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7193 mL | 8.5967 mL | 17.1934 mL | 34.3867 mL | 42.9834 mL |
| 5 mM | 0.3439 mL | 1.7193 mL | 3.4387 mL | 6.8773 mL | 8.5967 mL |
| 10 mM | 0.1719 mL | 0.8597 mL | 1.7193 mL | 3.4387 mL | 4.2983 mL |
| 50 mM | 0.0344 mL | 0.1719 mL | 0.3439 mL | 0.6877 mL | 0.8597 mL |
| 100 mM | 0.0172 mL | 0.086 mL | 0.1719 mL | 0.3439 mL | 0.4298 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bruton's tyrosine kinase (Btk) plays a key role in promoting B cell proliferation and survival through participation in the BCR signaling pathway. AVL-292 is a highly selective small molecule inhibitor of Btk currently being evaluated in a Phase 1b clinical trial.
In vitro: AVL-292 forms a covalent bond with Cys481 in Btk and potently inhibits Btk in biochemical (IC50 < 0.5nM) and cellular assays (EC50 1-10 nM) including α-IgM stimulation of BCR signaling, B cell proliferation and activation [1].
In vivo: Oral efficacy of AVL-292 in an established CIA model in mice was measured. The dose-dependent inhibition of the clinical signs of inflammatory disease was observed during the in-life portion of the model. In addition, all three AVL-292dose levels prevented the loss in body weight typically associated with severity of disease observed in this model [2].
Clinical trial: In healthy human volunteers, AVL-292 was found to be safe and well tolerated following oral administration at dose levels ranging from 0.5 to 7.0 mg/kg. AVL-292 pharmacokinetic and pharmacodynamic measurement of Btk engagement was dose-proportional across cohorts [1].
Reference:
[1] 53rd ASH Annual Meeting and Exposition. Abstract 3485: Clinical Development of AVL-292; A Potent, Selective Covalent Btk Inhibitor for the Treatment of B Cell Malignancies.
[2] Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, Witowski SR, Lounsbury H, Chaturvedi P, Mazdiyasni H, Zhu Z, Nacht M, Freed MI, Petter RC, Dubrovskiy A, Singh J, Westlin WF. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J Pharmacol Exp Ther. 2013;346(2):219-28.
- Ranaconitine
Catalog No.:BCN3870
CAS No.:1360-76-5
- Tetracaine HCl
Catalog No.:BCC4399
CAS No.:136-47-0
- Phenazopyridine HCl
Catalog No.:BCC4698
CAS No.:136-40-3
- Caulophylline B
Catalog No.:BCN7499
CAS No.:1359978-55-4
- SR 2211
Catalog No.:BCC6310
CAS No.:1359164-11-6
- RP 67580
Catalog No.:BCC7134
CAS No.:135911-02-3
- Lupinol C
Catalog No.:BCN4809
CAS No.:135905-53-2
- H-D-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2898
CAS No.:135904-71-1
- Blumenol C glucoside
Catalog No.:BCN6189
CAS No.:135820-80-3
- erythro-Guaiacylglycerol beta-dihydroconiferyl ether
Catalog No.:BCN7025
CAS No.:135820-77-8
- Koumine
Catalog No.:BCN6190
CAS No.:1358-76-5
- Isoforsythiaside
Catalog No.:BCN5413
CAS No.:1357910-26-9
- Angiotensin III (human, mouse)
Catalog No.:BCC1031
CAS No.:13602-53-4
- (1R,2S)-1-Amino-2-indanol
Catalog No.:BCC8381
CAS No.:136030-00-7
- Fmoc-Tic-OH
Catalog No.:BCC3341
CAS No.:136030-33-6
- Schisanlactone E
Catalog No.:BCN2593
CAS No.:136040-43-2
- Fmoc-Cys(pMeBzl)-OH
Catalog No.:BCC3476
CAS No.:136050-67-4
- Justiciresinol
Catalog No.:BCN3419
CAS No.:136051-41-7
- [D-Lys3]-GHRP-6
Catalog No.:BCC5850
CAS No.:136054-22-3
- Desrhamnosylmartynoside
Catalog No.:BCN7648
CAS No.:136055-64-6
- 9-Dehydroxyeurotinone
Catalog No.:BCN7397
CAS No.:1360606-85-4
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- 5-Methoxyisolariciresinol
Catalog No.:BCN7016
CAS No.:136082-41-2
- Onjixanthone II
Catalog No.:BCN7559
CAS No.:136083-93-7
A one-dimensional chloride-bridged Pt(II)/Pt(IV) mixed-valence complex with a 4-[(4-hydroxyphenyl)diazenyl]benzenesulfonate counter-ion.[Pubmed:26632826]
Acta Crystallogr C Struct Chem. 2015 Dec 1;71(Pt 12):1033-6.
The title compound, catena-poly[[[bis(ethylenediamine-kappa(2)N,N')platinum(II)]- mu-chlorido-[bis(ethylenediamine)platinum(IV)]-mu-chlorido] tetrakis{4-[(4-hydroxyphenyl)diazenyl]benzenesulfonate} dihydrate], {[Pt(II)Pt(IV)Cl2(C2H8N2)4](HOC6H4N=NC6H4SO3)4.2H2O}n, has a linear chain structure composed of square-planar [Pt(en)2](2+) (en is ethylenediamine) and elongated octahedral trans-[PtCl2(en)2](2+) cations stacked alternately, bridged by Cl atoms, along the b axis. The Pt atoms are located on an inversion centre, while the Cl atoms are disordered over two sites and form a zigzag ...Cl-Pt(IV)-Cl...Pt(II)... chain, with a Pt(IV)-Cl bond length of 2.3140 (14) A, an interatomic Pt(II)...Cl distance of 3.5969 (15) A and a Pt(IV)-Cl...Pt(II) angle of 170.66 (6) degrees . The structural parameter indicating the mixed-valence state of the Pt atom, expressed by delta = (Pt(IV)-Cl)/(Pt(II)...Cl), is 0.643.
Study of ciprofloxacin adsorption and regeneration of activated carbon prepared from Enteromorpha prolifera impregnated with H3PO4 and sodium benzenesulfonate.[Pubmed:28109901]
Ecotoxicol Environ Saf. 2017 May;139:36-42.
Activated carbons were derived from Enteromorpha prolifera immersed in H3PO4 solution or the H3PO4 solution mixed with sodium benzenesulfonate (SBS), producing AC and AC-SBS. NaOH solution was employed in regeneration of ciprofloxacin (CIP)-loaded AC and AC-SBS to obtain RAC and RAC-SBS. The properties of the original and regenerated activated carbons were characterized by thermo-gravimetric analysis (TGA), scanning electron microscopy (SEM), N2 adsorption/desorption isotherms and Fourier transform infrared spectroscopy (FTIR). Batched adsorption studies were carried out to compare CIP adsorption behaviors of the four carbons. The results suggested that the four samples exhibited higher proportions of mesopores and similar functional groups. Although AC displayed much higher specific surface area (SBET) (1045.79m(2)/g) than AC-SBS (738.03m(2)/g), its CIP adsorption capacity was much less than AC-SBS. The maximum adsorption capacity for AC, AC-SBS, RAC and RAC-SBS were found to be 250mg/g, 286mg/g, 233mg/g and 256mg/g, respectively, with the isotherms adhering to Langmuir isotherm model. The electrostatic attraction and cation exchange between CIP and the four carbons were the dominant adsorption mechanisms. Moreover, the thermodynamic parameters represented that the adsorption process had been confirmed to be a spontaneous and endothermic reaction.
Sulfonate salts of the therapeutic agent dapsone: 4-[(4-aminophenyl)sulfonyl]anilinium benzenesulfonate monohydrate and 4-[(4-aminophenyl)sulfonyl]anilinium methanesulfonate monohydrate.[Pubmed:27045177]
Acta Crystallogr C Struct Chem. 2016 Apr;72(Pt 4):280-4.
Dapsone, formerly used to treat leprosy, now has wider therapeutic applications. As is the case for many therapeutic agents, low aqueous solubility and high toxicity are the main problems associated with its use. Derivatization of its amino groups has been widely explored but shows no significant therapeutic improvements. Cocrystals have been prepared to understand not only its structural properties, but also its solubility and dissolution rate. Few salts of dapsone have been described. The title salts, C12H13N2O2S(+).C6H5O3S(-).H2O and C12H13N2O2S(+).CH3SO3(-).H2O, crystallize as hydrates and both compounds exhibit the same space group (monoclinic, P21/n). The asymmetric unit of each salt consists of a 4-[(4-aminophenyl)sulfonyl]anilinium monocation, the corresponding sulfonate anion and a water molecule. The cation, anion and water molecule form hydrogen-bonded networks through N-H...O=S, N-H...Owater and Owater-H...O=S hydrogen bonds. For both salts, the water molecules interact with one sulfonate anion and two anilinium cations. The benzenesulfonate salt forms a two-dimensional network, while the hydrogen bonding within the methanesulfonate salt results in a three-dimensional network.
Carbon dots as fluorescent probe for "off-on" Detecting sodium dodecyl-benzenesulfonate in aqueous solution.[Pubmed:26318701]
Spectrochim Acta A Mol Biomol Spectrosc. 2016 Jan 15;153:268-72.
In this paper, we propose an "off-on" approach for the detection of sodium dodecyl-benzenesulfonate (SDBS) using carbon dots (CDs) as fluorescent probe. We firstly demonstrated that the fluorescence of CDs decreased apparently in the presence of ruthenium (Ru), and the system was thus "turn-off". The resulting CDs-Ru system was found to be sensitive to SDBS, SDBS not only serves to shelter the CDs effectively from being quenched, but also to reverse the quenching and restore the fluorescence due to its ability to remove Ru from the surface of CDs (turn-on). An eco-friendly, simple and sensitive platform for the detection of SDBS based on the CDs-Ru probes has been proposed. After the experimental conditions were optimized, the linear range for detection SDBS was 0.10-7.50 mug/mL, with correlation coefficient (r) 0.9988, detection limit was 0.033 mug/mL (3sigma). This method is facile, rapid, low cost, environment-friendly, and possesses the potential for practical application.


