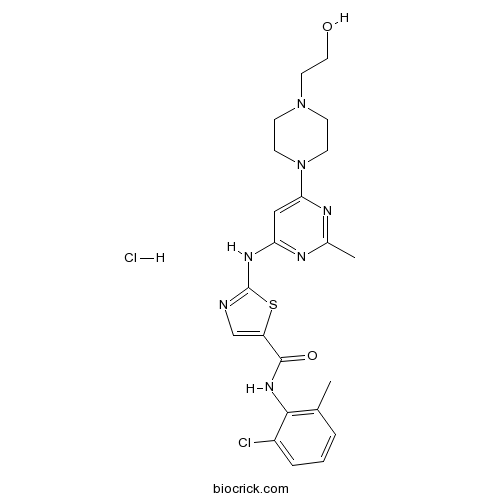
Dasatinib hydrochlorideMulti-BCR/Abl and Src kinase inhibitor, oral active CAS# 854001-07-3 |
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- A-770041
Catalog No.:BCC1323
CAS No.:869748-10-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 854001-07-3 | SDF | Download SDF |
| PubChem ID | 11466607 | Appearance | Powder |
| Formula | C22H27Cl2N7O2S | M.Wt | 524.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS 354825 hydrochloride | ||
| Solubility | DMSO : 15 mg/mL (28.60 mM; Need ultrasonic and warming) H2O : 10 mg/mL (19.07 mM; Need ultrasonic) | ||
| Chemical Name | N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide;hydrochloride | ||
| SMILES | CC1=C(C(=CC=C1)Cl)NC(=O)C2=CN=C(S2)NC3=NC(=NC(=C3)N4CCN(CC4)CCO)C.Cl | ||
| Standard InChIKey | MSCGWICDJYLQOJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H26ClN7O2S.ClH/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31;/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dasatinib hydrochloride is a potent and dual AblWT/Src inhibitor IC50 of 0.6 nM/0.8 nM respectively; also inhibits c-KitWT/c-KitD816V with IC50 of 79 nM/37 nM.In Vitro:Dasatinib potently inhibits wild-type Abl kinase and all mutants except T315I over a narrow range (IC50≤1.7 nM). Dasatinib (IC50: 0.8 nM) displays 325-fold greater potency compared with Imatinib against cells expressing wild-type Bcr-Abl in Ba/F3 cells[1].In Vivo:Daily treatment with Dasatinib (50 mg/kg) is initiated on day 10. Using this approach, a significant inhibition of BCPAP orthotopic tumor growth is observed 6 days after treatment (day 16, P=0.014), which is sustained through days 23 and 29 (P=0.0003), compared with vehicle-treated mice[3]. Metabolism studies of Dasatinib (50 mg/kg) in rat suggested that Dasatinib is the major circulating component, whereas multiple metabolites contributed to the remaining 40-60% of the sample radioactivity at 4 h post dose[4]. References: | |||||

Dasatinib hydrochloride Dilution Calculator

Dasatinib hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9067 mL | 9.5334 mL | 19.0669 mL | 38.1337 mL | 47.6672 mL |
| 5 mM | 0.3813 mL | 1.9067 mL | 3.8134 mL | 7.6267 mL | 9.5334 mL |
| 10 mM | 0.1907 mL | 0.9533 mL | 1.9067 mL | 3.8134 mL | 4.7667 mL |
| 50 mM | 0.0381 mL | 0.1907 mL | 0.3813 mL | 0.7627 mL | 0.9533 mL |
| 100 mM | 0.0191 mL | 0.0953 mL | 0.1907 mL | 0.3813 mL | 0.4767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Randomized trials have been conducted to compare the effects of dasatinib, nilotinib and imatinib in the treatment of newly diagnosed chronic myrloid leukemia in the chronic phase.
Abstract
Dasatinib is an inhibitor of tyrosine kinase including Src kinases that exhibits antitumor activity, affects osteoclasts and synergize with docetaxel. The efficacy of dasatinib alone or with docetaxel has been assessed in chemotherapy-naive men with metastatic castration-resistant prostate cancer.
Abstract
Dasatinib is a dual Abl/Src TKI that inhibits BCR-ABL with relatively greater potency and show potential immunomodulatory effects. Since it’s been approved to treat CML patients, the development of dasatinib is reviewed.
Abstract
Daily oral administration of dasatinib, an inhibitor of Src family kinases, to mice with breast cancer delays tumor onset and increases overall survival with the occurrence of squamous metaplasis accompanied by down-regulation of ErbB-2 and up-regulation of E-cadherin and β-catenin. Additionally, dasatinib inhibited both migration and invasion of tumour-derived cell lines in vitro.
Abstract
Dasatinib is an inhibitor of multiple tyrosine kinases that are used to treat CML and ALL. Although renal failure is a rare side effect of dasatinib, a patient with imatinib-resistant CML has developed both PE and ARF after receiving dastinib.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.6 nM (Abl); 0.8 nM (Src) [1] Dasatinib is an oral multi- BCR/Abl and Src family tyrosine kinase inhibitor approved for first line use in patients with chronic myelogenous leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia(Ph+ ALL). in vitro: Dasatinib potently inhibits wild-type Abl kinase and all mutants except T315I over a narrow range. Dasatinib directly targets wild-type and mutant Abl kinase domains and inhibits autophosphorylation and substrate phosphorylation in a concentration-dependent manner. Dasatinib displays 325-fold greater potency compared with imatinib against cells expressing wild-type Bcr-Abl [1]. DSB and radiotherapy induced a significant growth delay in both HNSCC xenograft models, although to a lesser extent in SCCNij202. DSB did not inhibit phosphorylated protein kinase B (pAKT) or phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) but did inhibit (phosphorylated) DNA-dependent protein kinase [2]. in vivo: Daily oral administration of dasatinib delays tumour onset and increases overall survival but does not inhibit the proliferation of established tumours. The striking difference between the dasatinib-treated group of tumours and the vehicle controls was the prominent squamous metaplasia that was seen in six out of 11 dasatinib-treated tumours [3]. In vivo studies were performed in a nude mouse xenograft model, the new prescription (DDP + Dasatinib) was better than DDP alone in terms of therapeutic efficacy [4]. Toxicity: Major cytogenetic response was significantly (P < 0.01) associated with weighted average steady-state dasatinib plasma concentrations, and pleural effusion was significantly associated with trough concentration [5]. We present a patient with chronic myelogenous leukemia who developed nephrotic-range proteinuria after initiation ondasatinib therapy that resolved after changing therapy to imatinib [6]. Clinical trial: Dasatinib, Cytarabine, and Idarubicin in Treating Patients With High-Risk Acute Myeloid Leukemia. Phase 1/Phase 2
- OGT 2115
Catalog No.:BCC7458
CAS No.:853929-59-6
- NVP-BAG956
Catalog No.:BCC1813
CAS No.:853910-02-8
- (+)-AJ 76 hydrochloride
Catalog No.:BCC6747
CAS No.:85378-82-1
- SKF 89976A hydrochloride
Catalog No.:BCC6930
CAS No.:85375-15-1
- 5,19-Epoxy-19R,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1328
CAS No.:85372-72-1
- 5,19-Epoxy-19S,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1329
CAS No.:85372-70-9
- SecinH3
Catalog No.:BCC7503
CAS No.:853625-60-2
- K03861
Catalog No.:BCC6537
CAS No.:853299-07-7
- Trichorabdal A
Catalog No.:BCN4404
CAS No.:85329-59-5
- Hythiemoside A
Catalog No.:BCN4403
CAS No.:853267-91-1
- Ajugalide D
Catalog No.:BCN3665
CAS No.:853247-65-1
- Ajugalide C
Catalog No.:BCN8015
CAS No.:853247-64-0
- Norcaesalpinin E
Catalog No.:BCN7006
CAS No.:854038-96-3
- (-)-Haplomyrfolin
Catalog No.:BCN3225
CAS No.:85404-48-4
- Ropivacaine mesylate
Catalog No.:BCC9137
CAS No.:854056-07-8
- Rilmenidine Phosphate
Catalog No.:BCC5637
CAS No.:85409-38-7
- S- (+)-Rolipram
Catalog No.:BCC2303
CAS No.:85416-73-5
- (R)-(-)-Rolipram
Catalog No.:BCC5429
CAS No.:85416-75-7
- Caffeic anhydride
Catalog No.:BCN3295
CAS No.:854237-32-4
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
- 4-Chlorotestosterone acetate
Catalog No.:BCC8705
CAS No.:855-19-6
- Eupatorin
Catalog No.:BCN4405
CAS No.:855-96-9
- PK 11195
Catalog No.:BCC6745
CAS No.:85532-75-8
- Safflor Yellow A
Catalog No.:BCN2408
CAS No.:85532-77-0
[Tyrosine kinase inhibitors and pregnancy: A risk to the fetus?].[Pubmed:26969425]
Bull Cancer. 2016 May;103(5):478-83.
The association of cancer and pregnancy is increasingly frequent. This is related, partially, to the increasingly belated age of pregnancy. The management of cancer in pregnancy is a complicated issue. The use of tyrosine kinase inhibitors (TKIs) during pregnancy remains rare and only few data are available concerning their transplacental passage. The aim of this work is to review the data described in the literature, in order to highlight the risks incurred by the fetus, associated with these TKIs' treatment. Up to 189 pregnancies of women treated with TKIs during part or throughout their pregnancy have been described. Clinical data are reassuring and would be in favor of taking the treatment in terms of the balance maternal profit versus fetal risk. These data must, nevertheless, be interpreted with caution.
CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma.[Pubmed:26855150]
Nat Med. 2016 Mar;22(3):278-87.
Although subtypes of pancreatic ductal adenocarcinoma (PDAC) have been described, this malignancy is clinically still treated as a single disease. Here we present patient-derived models representing the full spectrum of previously identified quasi-mesenchymal (QM-PDA), classical and exocrine-like PDAC subtypes, and identify two markers--HNF1A and KRT81--that enable stratification of tumors into different subtypes by using immunohistochemistry. Individuals with tumors of these subtypes showed substantial differences in overall survival, and their tumors differed in drug sensitivity, with the exocrine-like subtype being resistant to tyrosine kinase inhibitors and paclitaxel. Cytochrome P450 3A5 (CYP3A5) metabolizes these compounds in tumors of the exocrine-like subtype, and pharmacological or short hairpin RNA (shRNA)-mediated CYP3A5 inhibition sensitizes tumor cells to these drugs. Whereas hepatocyte nuclear factor 4, alpha (HNF4A) controls basal expression of CYP3A5, drug-induced CYP3A5 upregulation is mediated by the nuclear receptor NR1I2. CYP3A5 also contributes to acquired drug resistance in QM-PDA and classical PDAC, and it is highly expressed in several additional malignancies. These findings designate CYP3A5 as a predictor of therapy response and as a tumor cell-autonomous detoxification mechanism that must be overcome to prevent drug resistance.
Impact of Small Molecules on beta-Catenin and E-Cadherin Expression in HPV16-positive and -negative Squamous Cell Carcinomas.[Pubmed:28551620]
Anticancer Res. 2017 Jun;37(6):2845-2852.
BACKGROUND: The validation of potential molecular targets in head and neck squamous cell carcinoma (SCC) is mandatory. beta-Catenin and E-cadherin are crucial for cancer progression through epithelial-mesenchymal transition. We analyzed the effect of the tyrosine kinase inhibitors nilotinib, dasatinib, erlotinib and gefitinib on beta-catenin and E-cadherin expression in SCC with respect to human papillomavirus (HPV) status. MATERIALS AND METHODS: Expression of beta-catenin and E-cadherin in cell lines UMSCC 11A, UMSCC 14C and CERV196 under the influence of tyrosine kinase inhibitors were analyzed by enzyme-linked immunosorbent assay. RESULTS: All agents reduced beta-catenin and E-cadherin expression of HPV16-negative cells. Increased E-cadherin expression was observed after treatment with gefitinib and dasatinib in HPV16-positive cells. CONCLUSION: All substances, nilotinib, dasatinib, erlotinib and gefitinib have a significant impact on beta-catenin and E-cadherin expression in both HPV16-positive and HPV16-negative cells in vitro. Alterations of beta-catenin and E-cadherin could provide novel insights for future targeted therapies of head and neck SCC.
Overview of Current Treatment Options and Investigational Targeted Therapies for Locally Advanced Squamous Cell Carcinoma of the Head and Neck.[Pubmed:26967327]
Am J Clin Oncol. 2016 Aug;39(4):396-406.
Patients with squamous cell carcinoma of the head and neck (SCCHN) typically present with locally advanced (LA) stage III or IV disease and are treated with combined-modality therapy with chemotherapy, radiotherapy, and surgery (if resectable). These aggressive, upfront treatment measures are often associated with substantial morbidity, and about half the patients develop locoregional or distant recurrences. Thus, new therapeutic strategies are needed that offer similar efficacy benefits with less toxicity. Current research is focused on selectively targeting signaling pathways involved in the proliferation and malignant transformation of SCCHN cells and the tumor microenvironment. For example, the ErbB receptor pathway has been implicated in the development and progression of SCCHN, and several agents targeting this pathway and downstream effectors are in various phases of clinical investigation. Cetuximab, a monoclonal antibody against epidermal growth factor receptor (EGFR), is the only currently approved targeted therapy for the treatment of LA SCCHN. Additional agents targeting EGFR and other ErbB family members, including monoclonal antibodies (eg, panitumumab, nimotuzumab) and small-molecule tyrosine kinase inhibitors (eg, erlotinib, afatinib, lapatinib) are being studied in LA SCCHN with varying results. Other treatment strategies for LA SCCHN include targeting downstream effectors of signaling and resistance mechanisms to EGFR inhibitors (eg, mammalian target of rapamycin, Src family, and Aurora kinase family). Data from ongoing and future clinical trials will continue to refine current treatment paradigms for LA SCCHN and provide new therapeutic options and potential predictive biomarkers to improve patient efficacy and safety and abrogate resistance.
A streamlined search technology for identification of synergistic drug combinations.[Pubmed:26416286]
Sci Rep. 2015 Sep 29;5:14508.
A major key to improvement of cancer therapy is the combination of drugs. Mixing drugs that already exist on the market may offer an attractive alternative. Here we report on a new model-based streamlined feedback system control (s-FSC) method, based on a design of experiment approach, for rapidly finding optimal drug mixtures with minimal experimental effort. We tested combinations in an in vitro assay for the viability of a renal cell adenocarcinoma (RCC) cell line, 786-O. An iterative cycle of in vitro testing and s-FSC analysis was repeated a few times until an optimal low dose combination was reached. Starting with ten drugs that target parallel pathways known to play a role in the development and progression of RCC, we identified the best overall drug combination, being a mixture of four drugs (axitinib, erlotinib, dasatinib and AZD4547) at low doses, inhibiting 90% of cell viability. The removal of AZD4547 from the optimized drug combination resulted in 80% of cell viability inhibition, while still maintaining the synergistic interaction. These optimized drug combinations were significantly more potent than monotherapies of all individual drugs (p < 0.001, CI < 0.3).


