SC 19220Selective EP1 receptor antagonist CAS# 19395-87-0 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- Grape Seed Extract
Catalog No.:BCC5317
CAS No.:84929-27-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

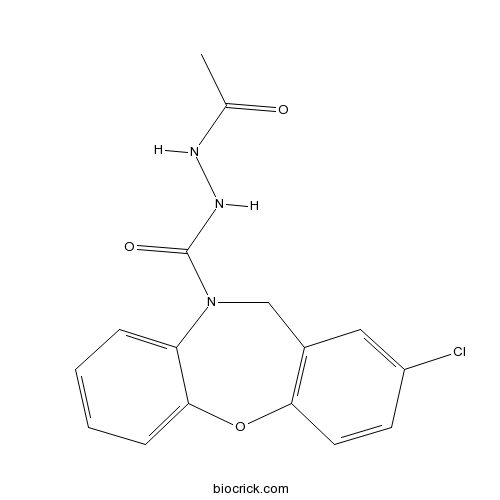
| Cas No. | 19395-87-0 | SDF | Download SDF |
| PubChem ID | 29569 | Appearance | Powder |
| Formula | C16H14ClN3O3 | M.Wt | 331.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in ethanol with gentle warming and to 100 mM in DMSO | ||
| Chemical Name | N'-acetyl-8-chloro-6H-benzo[b][1,4]benzoxazepine-5-carbohydrazide | ||
| SMILES | CC(=O)NNC(=O)N1CC2=C(C=CC(=C2)Cl)OC3=CC=CC=C31 | ||
| Standard InChIKey | UETSVHKFTALXNP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14ClN3O3/c1-10(21)18-19-16(22)20-9-11-8-12(17)6-7-14(11)23-15-5-3-2-4-13(15)20/h2-8H,9H2,1H3,(H,18,21)(H,19,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective EP1 receptor antagonist (IC50 = 6.7 μM for inhibition of [3H]-PGE2 binding to EP1 transfected COS cells). |

SC 19220 Dilution Calculator

SC 19220 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0142 mL | 15.0711 mL | 30.1423 mL | 60.2845 mL | 75.3557 mL |
| 5 mM | 0.6028 mL | 3.0142 mL | 6.0285 mL | 12.0569 mL | 15.0711 mL |
| 10 mM | 0.3014 mL | 1.5071 mL | 3.0142 mL | 6.0285 mL | 7.5356 mL |
| 50 mM | 0.0603 mL | 0.3014 mL | 0.6028 mL | 1.2057 mL | 1.5071 mL |
| 100 mM | 0.0301 mL | 0.1507 mL | 0.3014 mL | 0.6028 mL | 0.7536 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Phenyl-1,2-dihydroacenaphthylene-1,2-diol
Catalog No.:BCN7178
CAS No.:193892-33-0
- Fmoc-β-Homo-Leu-OH
Catalog No.:BCC2631
CAS No.:193887-44-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Cortistatin 14
Catalog No.:BCC6010
CAS No.:193829-96-8
- SB 242235
Catalog No.:BCC4171
CAS No.:193746-75-7
- Aminoguanidine hydrochloride
Catalog No.:BCC6795
CAS No.:1937-19-5
- 8beta-Methoxyatractylenolide I
Catalog No.:BCN7594
CAS No.:193694-24-5
- Methyl 4-hydroxycinnamate
Catalog No.:BCN4014
CAS No.:19367-38-5
- TAS 301
Catalog No.:BCC6214
CAS No.:193620-69-8
- BRL-15572
Catalog No.:BCC5065
CAS No.:193611-72-2
- SB 216641 hydrochloride
Catalog No.:BCC6987
CAS No.:193611-67-5
- Terrestrosin K
Catalog No.:BCN2935
CAS No.:193605-07-1
- Fmoc-ß-HoAla-OH
Catalog No.:BCC3225
CAS No.:193954-26-6
- Fmoc- ß-HoIle-OH
Catalog No.:BCC3237
CAS No.:193954-27-7
- Fmoc-β-HoPhe-OH
Catalog No.:BCC3241
CAS No.:193954-28-8
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- Dihydrocapsaicin
Catalog No.:BCN1017
CAS No.:19408-84-5
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
- Isosaponarin
Catalog No.:BCN2279
CAS No.:19416-87-6
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
SC-19220, antagonist of prostaglandin E2 receptor EP1, inhibits osteoclastogenesis by RANKL.[Pubmed:15619665]
J Bone Miner Res. 2005 Jan;20(1):15-22.
UNLABELLED: We examined the direct effect of SC-19220, an EP1 prostaglandin (PG) E2 receptor antagonist, on osteoclastogenesis induced by RANK/RANKL signaling in mouse cell cultures. We found that SC-19220 inhibited RANKL-induced osteoclastogenesis by suppression of the RANK/RANKL signaling pathway in osteoclast precursors. INTRODUCTION: Bone growth is accomplished by a dynamic equilibrium between formation by osteoblasts and resorption by osteoclasts, which are regulated by many systemic and local osteotropic factors that induce osteoclast formation from hematopoietic precursors through RANK/RANKL signaling. There are four subtypes of prostaglandin E (PGE) receptors, EP1, EP2, EP3, and EP4, and PGE2 facilitates bone resorption by a mechanism mediated by EP2/EP4. It is well known that SC-19220 is an EP1-specific antagonist. We previously found that SC-19220 inhibited osteoclastogenesis induced by osteotropic factors, including PGE2; however, the inhibitory mechanism is not clear. In this study, we investigated the inhibitory effects of SC-19220 on osteoclastogenesis induced by RANK/RANKL signaling in mouse cell cultures and analyzed the mechanism involved. MATERIALS AND METHODS: A bone marrow culture system and bone marrow macrophages were used to examine the effects of SC-19220 on PGE2-, 11-deoxy-PGE1-, and RANKL-induced osteoclastogenesis. We analyzed RANKL expression in osteoblasts induced by PGE2 using RT-PCR. We also examined the effects of SC-19220 on the macrophage-colony-stimulating factor (M-CSF) receptor (c-Fms) and RANK expression in osteoclast precursors as well as RANK/RANKL signaling using RT-PCR and Western blotting analyses. RESULTS AND CONCLUSION: SC-19220 dose-dependently inhibited osteoclast formation induced by PGE2, 11-deoxy-PGE1, and RANKL in the mouse culture system; however, it had no influence on RANKL expression in osteoblasts induced by PGE2. Furthermore, the expression of RANK and c-Fms in osteoclast precursors was decreased by SC-19220 at the mRNA and protein levels. In RANK signaling networks, SC-19220 inhibited c-Src and NFAT2 expression. Our findings indicated that SC-19220 inhibits RANKL-induced osteoclastogenesis through the suppression of RANK, c-Fms, c-Src, and NFAT2, suggesting that this EP1-specific antagonist inhibits osteoclast formation induced by RANKL from the early stage of osteoclastogenesis.
Prostaglandin E2 receptor antagonist (SC-19220) treatment restores the balance to bone marrow myelopoiesis after burn sepsis.[Pubmed:10823525]
J Trauma. 2000 May;48(5):826-30; discussion 830-1.
BACKGROUND: Although prostaglandin E2 (PGE2) has been shown to be immunosuppressive, its role in the development of specific bone marrow myeloid lineages after thermal injury and sepsis has yet to be elucidated. The purpose of this study was to demonstrate that alterations in bone marrow progenitor proliferation favoring monocytopoiesis in burn sepsis can be restored by blocking the cellular interactions of PGE2. METHODS: A murine model of burn sepsis with and without treatment with SC-19220, a PGE2 receptor antagonist, was used to determine peripheral monocyte and neutrophil counts as well as the colony forming potential of colony-stimulating factor responsive bone marrow progenitors. RESULTS: Burn sepsis augmented the growth of the early colony-forming unit granulocyte-macrophage and monocyte progenitors and the number of circulating monocytes, whereas granulocyte progenitors and circulating neutrophils demonstrated an opposite response. Treatment with SC-19220 nearly reversed these alterations. CONCLUSION: These data indicate that abrogating PGE2's actions during burn sepsis can restore the balance in bone marrow granulocyte and monocyte production, further consolidating the pivotal role PGE2 plays in the pathogenesis of burn sepsis.
Contractile effects of prostaglandin E2 in rat rectum: sensitivity to the prostaglandin antagonists diphloretin phosphate and SC 19220.[Pubmed:1361677]
Prostaglandins. 1992 Nov;44(5):471-83.
Prostaglandin E2 (PGE2) applied cumulatively (1 nM-1 microM) induced concentration-dependent tonic contractions in the longitudinal muscle of isolated rat rectum. The PGE2 effects were not altered by guanethidine (50 microM), whereas atropine (3 microM), guanethidine plus atropine or tetrodotoxin (0.1 microM) reduced them to an almost equal extent and increased the EC50 values for PGE2. The after-contractions following electrical stimulation were enhanced by PGE2 (10 nM) and inhibited by atropine. Diphloretin phosphate (DPP, 100 microM) shifted the regression lines for PGE2 to the right in both untreated and tetrodotoxin-treated preparations, and thereby increased the EC50 values. Slopes of the concentration-effect lines for PGE2 before and after DPP differed in the presence of tetrodotoxin. The regression line for PGE2 with SC 19220 (100 microM) in tetrodotoxin-treated preparations was shifted to the right in a parallel fashion. It is concluded that PGE2 exerted both a neural (cholinergic) and a smooth muscle effect. There may be a competitive antagonism between SC 19220 and PGE2 but the block by DPP may be nonselective.
SC-19220, a prostaglandin E2 antagonist, inhibits osteoclast formation by 1,25-dihydroxyvitamin D3 in cell cultures.[Pubmed:10320820]
J Endocrinol. 1999 May;161(2):231-6.
1,25 Dihydroxy vitamin D3 (1,25(OH)2D3), prostaglandin (PG) E2 and parathyroid hormone (PTH) induce osteoclast formation in cell cultures. Previously, we have shown that SC-19220, an antagonist of the EP1 subtype of PGE receptors, inhibited tartrate-resistant acid phosphatase (TRAP)-positive cell formation by PGE2 and PTH in adherent cell cultures taken from neonatal rats. Since 1,25(OH)2D3 has been shown to induce osteoclast formation through PGE2 synthesis, in this study we have examined the effect of SC-19220 on osteoclast formation induced by 1,25(OH)2D3 in cell cultures by measuring bone resorption as well as TRAP-positive cell formation. SC-19220 inhibited osteoclast formation by 1,25(OH)2D3 as well as by PGE2 in cell cultures. The addition of SC-19220 to the later half but not to the earlier half of the culture inhibited 1,25(OH)2D3-induced formation. In the culture in which hydroxyurea was added in the later half period, SC-19220 inhibited osteoclast formation by 1, 25(OH)2D3. Under these conditions, 17-phenyl PGE1, an EP1 agonist, induced osteoclast formation. Thus, SC-19220 inhibits certain reactions in the later processes of osteoclast formation induced by 1,25(OH)2D3. In addition, SC-19220 also inhibited osteoclast formation induced by interleukin (IL)-11 and IL-6 as well as by PTH. It is suggested that the SC-19220 inhibiting reactions are shared by all the inducers including 1,25(OH)2D3 and are essential for osteoclast formation.
Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon.[Pubmed:7791070]
J Pharmacol Exp Ther. 1995 Jun;273(3):1008-14.
Smooth muscle strips and isolated muscle cells from the circular layer of dog colon, were used to study the effect of prostaglandin E2 (PGE2) and their analogs acting at EP receptors: Iloprost (IP/EP1), butaprost (EP2) and enprostil (EP3) and SC19220 (antagonist EP1) to characterize the EP-receptors involved in the control of muscle function. In strips treated with tetrodotoxin, only enprostil provoked a concentration-dependent contraction. The concentration of enprostil inducing a half maximal contraction (EC50) was 400 nM and the maximal effect was obtained at 1 microM. PGE2, butaprost and iloprost induced a dose-dependent relaxation, with an EC50 of 200, 80 and 200 nM, respectively. The maximal relaxation was obtained at 1 microM for all these agents. When the EP1 antagonist, SC19220 (10 microM), was added 20 min before PGE2 or their analogs, their respective concentration-response curves were not affected. In isolated cells, PGE2 and enprostil induced a cell contraction in a concentration-dependent manner, whereas iloprost and butaprost had no effect by themselves. The maximal contraction was 241.1 +/- 1.7% at 10 nM PGE2 and 22.5 +/- 1.6% at 10 nM enprostil. EC50 of PGE2 and enprostil was 40 pM. SC 19220, at concentrations ranging from 1 pM to 0.1 microM, failed to inhibit the contraction induced by either PGE2 or enprostil. When cells were preincubated for 1 min with butaprost or iloprost at concentrations ranging from 1 pM to 1 microM, the contraction induced by CCK8 (10nM) was inhibited in a concentration-dependent matter.(ABSTRACT TRUNCATED AT 250 WORDS)
Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype.[Pubmed:8253813]
J Biol Chem. 1993 Dec 15;268(35):26767-72.
A functional cDNA clone coding for the human prostaglandin E receptor EP1 subtype has been isolated from a human erythroleukemia cell cDNA library probed by low-stringency hybridization using a polymerase chain reaction fragment of the human thromboxane receptor. The human EP1 receptor is comprised of 402 amino acids with a predicted molecular mass of 41,858 and has the topography common to all G-protein-coupled receptors with seven predicted transmembrane spanning domains. Prostaglandin (PG) E2 challenge of Xenopus oocytes injected with EP1 cDNA resulted in an increase in intracellular Ca2+. In addition, the rank order of potency for prostaglandins in competition for [3H]PGE2 specific binding to membranes prepared from EP1 cDNA transfected COS cells was PGE2 > PGE1 > PGF2 alpha > PGD2. Furthermore, the EP1 receptor-selective antagonists AH 6809 and SC19220 were more potent than the EP2 receptor-selective agonist butaprost in these competition binding assays. In summary, therefore, we have cloned the human EP1 receptor subtype which is functionally coupled to an increase in intracellular Ca2+.


