DL-5-HydroxytryptophanCAS# 56-69-9 |
- L-5-Hydroxytryptophan
Catalog No.:BCC8106
CAS No.:4350-09-8
Quality Control & MSDS
Number of papers citing our products

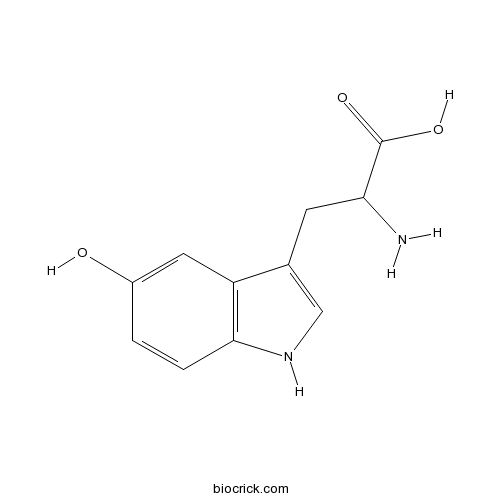
Chemical structure

3D structure
| Cas No. | 56-69-9 | SDF | Download SDF |
| PubChem ID | 144 | Appearance | White powder |
| Formula | C11H12N2O3 | M.Wt | 220.23 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxy-DL-tryptophan | ||
| Solubility | Soluble to 44 mg/mL (199.8 mM) in DMSO | ||
| Chemical Name | 2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid | ||
| SMILES | C1=CC2=C(C=C1O)C(=CN2)CC(C(=O)O)N | ||
| Standard InChIKey | LDCYZAJDBXYCGN-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-Hydroxytryptophan (5-HTP) is the precursor of serotonin , has been widely used as a dietary supplement to raise serotonin level, it is an effective drug against depression, insomnia, obesity, chronic headaches, etc. 5-HTP supplementation can inhibit endothelial serotonylation, leukocyte recruitment, and allergic inflammation, it also can reduce the symptoms of anxiety/depression of that patients with allergy/asthma. 5-HTP may be involved in inducing the female to stay in copula and delay egg-laying to prevent re-mating of the female. |
| Targets | NF-kB | IL Receptor | TNF-α |
| In vitro | Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan[Pubmed: 22842218]Am J Physiol Lung Cell Mol Physiol. 2012 Oct 15; 303(8): L642–L660.Clinical reports indicate that patients with allergy/asthma commonly have associated symptoms of anxiety/depression. Anxiety/depression can be reduced by 5-Hydroxytryptophan (5-HTP) supplementation. However, it is not known whether 5-HTP reduces allergic inflammation. Therefore, we determined whether 5-HTP supplementation reduces allergic inflammation. We also determined whether 5-HTP decreases passage of leukocytes through the endothelial barrier by regulating endothelial cell function. For these studies, C57BL/6 mice were supplemented with 5-HTP, treated with ovalbumin fraction V (OVA), house dust mite (HDM) extract, or IL-4, and examined for allergic lung inflammation and OVA-induced airway responsiveness.
|
| In vivo | Oral administration of 5-hydroxytryptophan aggravated periodontitis-induced alveolar bone loss in rats.[Pubmed: 25766472]Arch Oral Biol. 2015 May;60(5):789-98.5-Hydroxytryptophan (5-HTP) is the precursor of serotonin and 5-Hydroxytryptophan has been widely used as a dietary supplement to raise serotonin level. Serotonin has recently been discovered to be a novel and important player in bone metabolism. As peripheral serotonin negatively regulates bone, the regular take of 5-Hydroxytryptophan may affect the alveolar bone metabolism and therefore influence the alveolar bone loss induced by periodontitis. The aim of this study was to investigate the effect of 5-Hydroxytryptophan on alveolar bone destruction in periodontitis. |
| Animal Research | Male-to-female transfer of 5-hydroxytryptophan glucoside during mating in Zygaena filipendulae (Lepidoptera).[Pubmed: 24012995]Insect Biochem Mol Biol. 2013 Nov;43(11):1037-44.Zygaena filipendulae accumulates the cyanogenic glucosides linamarin and lotaustralin by larval sequestration from the food plant or de novo biosynthesis. We have previously demonstrated that the Z. filipendulae male transfers linamarin and lotaustralin to the female in the course of mating.
|
| Structure Identification | ACS Synth Biol. 2014 Jul 18;3(7):497-505.Engineering bacterial phenylalanine 4-hydroxylase for microbial synthesis of human neurotransmitter precursor 5-hydroxytryptophan.[Pubmed: 24936877]5-Hydroxytryptophan (5-HTP) is a drug that is clinically effective against depression, insomnia, obesity, chronic headaches, etc. It is only commercially produced by the extraction from the seeds of Griffonia simplicifolia because of a lack of synthetic methods.
|

DL-5-Hydroxytryptophan Dilution Calculator

DL-5-Hydroxytryptophan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5407 mL | 22.7035 mL | 45.4071 mL | 90.8141 mL | 113.5177 mL |
| 5 mM | 0.9081 mL | 4.5407 mL | 9.0814 mL | 18.1628 mL | 22.7035 mL |
| 10 mM | 0.4541 mL | 2.2704 mL | 4.5407 mL | 9.0814 mL | 11.3518 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9081 mL | 1.8163 mL | 2.2704 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9081 mL | 1.1352 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quinidine
Catalog No.:BCC7863
CAS No.:56-54-2
- Diethylstilbestrol
Catalog No.:BCC4900
CAS No.:56-53-1
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
- H-Gly-OH
Catalog No.:BCC2946
CAS No.:56-40-6
- Tetraethylammonium chloride
Catalog No.:BCC7554
CAS No.:56-34-8
- Cantharidin
Catalog No.:BCN1280
CAS No.:56-25-7
- Cystamine dihydrochloride
Catalog No.:BCC6344
CAS No.:56-17-7
- 4-Aminobutanoic acid
Catalog No.:BCN2187
CAS No.:56-12-2
- 2,4-Diamino-6-hydroxypyrimidine
Catalog No.:BCC6658
CAS No.:56-06-4
- Methylthiouracil
Catalog No.:BCC4800
CAS No.:56-04-2
- Chloramphenicol
Catalog No.:BCC1201
CAS No.:56-75-7
- Glycerol
Catalog No.:BCC8990
CAS No.:56-81-5
- H-Asp-OH
Catalog No.:BCC2881
CAS No.:56-84-8
- L-Glutamine
Catalog No.:BCC3803
CAS No.:56-85-9
- L-Glutamic acid
Catalog No.:BCN3809
CAS No.:56-86-0
- L-lysine
Catalog No.:BCN7157
CAS No.:56-87-1
- (H-Cys-OH)2
Catalog No.:BCC2915
CAS No.:56-89-3
- Histamine 2HCl
Catalog No.:BCC4530
CAS No.:56-92-8
- Chlorhexidine acetate
Catalog No.:BCC8912
CAS No.:56-95-1
- 9-Hydroxy-4-androstene-3,17-dione
Catalog No.:BCC8802
CAS No.:560-62-3
- Eburicoic acid
Catalog No.:BCN2556
CAS No.:560-66-7
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
Oral administration of 5-hydroxytryptophan aggravated periodontitis-induced alveolar bone loss in rats.[Pubmed:25766472]
Arch Oral Biol. 2015 May;60(5):789-98.
OBJECTIVE: 5-Hydroxytryptophan (5-HTP) is the precursor of serotonin and 5-HTP has been widely used as a dietary supplement to raise serotonin level. Serotonin has recently been discovered to be a novel and important player in bone metabolism. As peripheral serotonin negatively regulates bone, the regular take of 5-HTP may affect the alveolar bone metabolism and therefore influence the alveolar bone loss induced by periodontitis. The aim of this study was to investigate the effect of 5-HTP on alveolar bone destruction in periodontitis. DESIGN: Male Sprague-Dawley rats were randomly divided into the following four groups: (1) the control group (without ligature); (2) the 5-HTP group (5-HTP at 25 mg/kg/day without ligature); (3) the L group (ligature+saline placebo); and (4) the L+5-HTP group (ligature+5-HTP at 25 mg/kg/day). Serum serotonin levels were determined by ELISA. The alveolar bones were evaluated with micro-computed tomography and histology. Tartrate-resistant acid phosphatase staining was used to assess osteoclastogenesis. The receptor activator of NF-kB ligand (RANKL) and osteoprotegerin (OPG) expression in the periodontium as well as the interleukin-6 positive osteocytes were analysed immunohistochemically. RESULTS: 5-HTP significantly increased serum serotonin levels. In rats with experimental periodontitis, 5-HTP increased alveolar bone resorption and worsened the micro-structural destruction of the alveolar bone. 5-HTP also stimulated osteoclastogenesis and increased RANKL/OPG ratio and the number of IL-6 positive osteocytes. However, 5-HTP treatment alone did not cause alveolar bone loss in healthy rats. CONCLUSION: The present study showed that 5-HTP aggravated alveolar bone loss, deteriorated alveolar bone micro-structure in the presence of periodontitis, which suggests 5-HTP administration may increase the severity of periodontitis.
Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan.[Pubmed:22842218]
Am J Physiol Lung Cell Mol Physiol. 2012 Oct 15;303(8):L642-60.
Clinical reports indicate that patients with allergy/asthma commonly have associated symptoms of anxiety/depression. Anxiety/depression can be reduced by 5-hydroxytryptophan (5-HTP) supplementation. However, it is not known whether 5-HTP reduces allergic inflammation. Therefore, we determined whether 5-HTP supplementation reduces allergic inflammation. We also determined whether 5-HTP decreases passage of leukocytes through the endothelial barrier by regulating endothelial cell function. For these studies, C57BL/6 mice were supplemented with 5-HTP, treated with ovalbumin fraction V (OVA), house dust mite (HDM) extract, or IL-4, and examined for allergic lung inflammation and OVA-induced airway responsiveness. To determine whether 5-HTP reduces leukocyte or eosinophil transendothelial migration, endothelial cells were pretreated with 5-HTP, washed and then used in an in vitro transendothelial migration assay under laminar flow. Interestingly, 5-HTP reduced allergic lung inflammation by 70-90% and reduced antigen-induced airway responsiveness without affecting body weight, blood eosinophils, cytokines, or chemokines. 5-HTP reduced allergen-induced transglutaminase 2 (TG2) expression and serotonylation (serotonin conjugation to proteins) in lung endothelial cells. Consistent with the regulation of endothelial serotonylation in vivo, in vitro pretreatment of endothelial cells with 5-HTP reduced TNF-alpha-induced endothelial cell serotonylation and reduced leukocyte transendothelial migration. Furthermore, eosinophil and leukocyte transendothelial migration was reduced by inhibitors of transglutaminase and by inhibition of endothelial cell serotonin synthesis, suggesting that endothelial cell serotonylation is key for leukocyte transendothelial migration. In summary, 5-HTP supplementation inhibits endothelial serotonylation, leukocyte recruitment, and allergic inflammation. These data identify novel potential targets for intervention in allergy/asthma.
Engineering bacterial phenylalanine 4-hydroxylase for microbial synthesis of human neurotransmitter precursor 5-hydroxytryptophan.[Pubmed:24936877]
ACS Synth Biol. 2014 Jul 18;3(7):497-505.
5-Hydroxytryptophan (5-HTP) is a drug that is clinically effective against depression, insomnia, obesity, chronic headaches, etc. It is only commercially produced by the extraction from the seeds of Griffonia simplicifolia because of a lack of synthetic methods. Here, we report the efficient microbial production of 5-HTP via combinatorial protein and metabolic engineering approaches. First, we reconstituted and screened prokaryotic phenylalanine 4-hydroxylase activity in Escherichia coli. Then, sequence- and structure-based protein engineering dramatically shifted its substrate preference, allowing for efficient conversion of tryptophan to 5-HTP. Importantly, E. coli endogenous tetrahydromonapterin (MH4) could be utilized as the coenzyme, when a foreign MH4 recycling mechanism was introduced. Whole-cell bioconversion allowed the high-level production of 5-HTP (1.1-1.2 g/L) from tryptophan in shake flasks. On this basis, metabolic engineering efforts were further made to achieve the de novo 5-HTP biosynthesis from glucose. This work not only holds great scale-up potential but also demonstrates a strategy for expanding the native metabolism of microorganisms.
Emitting state of 5-hydroxyindole, 5-hydroxytryptophan, and 5-hydroxytryptophan incorporated in proteins.[Pubmed:24020960]
J Phys Chem B. 2013 Sep 19;117(37):10792-7.
5-Hydroxy-L-tryptophan (5HW) has been biosynthetically incorporated in many proteins to facilitate their characterization using fluorescence spectroscopy. An attractive feature of this tryptophan analogue is its absorbance at 310-320 nm, allowing its specific excitation in a Trp background. The red-shift in absorbance upon introduction of a hydroxyl group at the 5-position of Trp or indole was found to be due to a lowering of the (1)Lb transition energy. It was therefore believed that 5HW only features (1)Lb emission. Recently, calculations for 5-hydroxyindole (5HI) in water revealed (1)La is the emitting state, and the same was predicted for 5HW incorporated in proteins. To clarify which state emits in 5HI and 5HW, we present here excitation anisotropy spectra of these probes and of four proteins labeled with 5HW at a surface exposed position. Our data clearly show (1)Lb is the emitting state of 5HI, 5HW, and 5HW in three of the proteins investigated. For one protein mixed emission was observed, and the decay kinetics were found strongly dependent on the emission wavelength. This work provides the first experimental evidence that (1)La can be the emitting state for this Trp analogue incorporated in a protein.
Male-to-female transfer of 5-hydroxytryptophan glucoside during mating in Zygaena filipendulae (Lepidoptera).[Pubmed:24012995]
Insect Biochem Mol Biol. 2013 Nov;43(11):1037-44.
Zygaena filipendulae accumulates the cyanogenic glucosides linamarin and lotaustralin by larval sequestration from the food plant or de novo biosynthesis. We have previously demonstrated that the Z. filipendulae male transfers linamarin and lotaustralin to the female in the course of mating. In this study we report the additional transfer of 5-hydroxytryptophan glucoside (5-(beta-d-glucopyranosyloxy)-L-Tryptophan) from the Z. filipendulae male internal genitalia to the female spermatophore around 5 h into the mating process. 5-Hydroxytryptophan glucoside is present in the virgin male internal genitalia, and production continues during the early phase of mating. Following initiation of 5-hydroxytryptophan glucoside transfer to the female, the amount in male internal genitalia is drastically reduced until after mating where it is slowly replenished. For unambiguous structural identification, 5-hydroxytryptophan glucoside was chemically synthesized and used as an authentic standard. The biological function of 5-hydroxytryptophan glucoside remains to be established, although we have indications that it may be involved in inducing the female to stay in copula and delay egg-laying to prevent re-mating of the female. To our knowledge 5-hydroxytryptophan glucoside has not previously been reported present in animal tissues.


