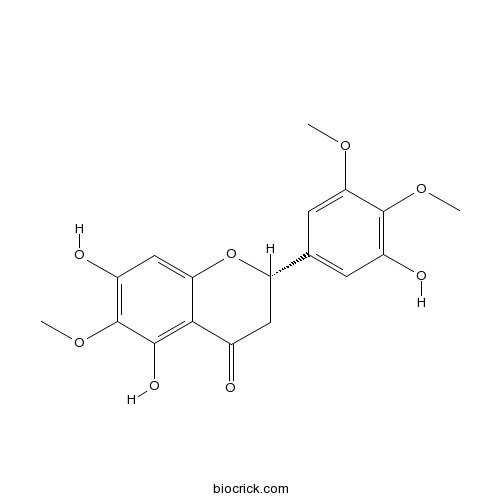
5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanoneCAS# 310888-07-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 310888-07-4 | SDF | Download SDF |
| PubChem ID | 71463648 | Appearance | Powder |
| Formula | C18H18O8 | M.Wt | 362.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-5,7-dihydroxy-2-(3-hydroxy-4,5-dimethoxyphenyl)-6-methoxy-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1OC)O)C2CC(=O)C3=C(C(=C(C=C3O2)O)OC)O | ||
| Standard InChIKey | HMTSHCGCQPCGLA-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C18H18O8/c1-23-14-5-8(4-10(20)17(14)24-2)12-6-9(19)15-13(26-12)7-11(21)18(25-3)16(15)22/h4-5,7,12,20-22H,6H2,1-3H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Journal of Natural Products, 2000, 63(12):1689-1691.Two Novel Flavanones from Greigia sphacelata.[Reference: WebLink]As part of our continuing phytochemical investigations of plants from arid environments in Chile, the aerial parts of Greigia sphacelata were examined. |

5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone Dilution Calculator

5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7601 mL | 13.8007 mL | 27.6014 mL | 55.2029 mL | 69.0036 mL |
| 5 mM | 0.552 mL | 2.7601 mL | 5.5203 mL | 11.0406 mL | 13.8007 mL |
| 10 mM | 0.276 mL | 1.3801 mL | 2.7601 mL | 5.5203 mL | 6.9004 mL |
| 50 mM | 0.0552 mL | 0.276 mL | 0.552 mL | 1.1041 mL | 1.3801 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.276 mL | 0.552 mL | 0.69 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PRT 4165
Catalog No.:BCC6354
CAS No.:31083-55-3
- Decylic acid vanillylamide
Catalog No.:BCN7836
CAS No.:31078-36-1
- Alytesin
Catalog No.:BCC7203
CAS No.:31078-12-3
- Cedeodarin
Catalog No.:BCN4784
CAS No.:31076-39-8
- Spaglumic acid
Catalog No.:BCC6632
CAS No.:3106-85-2
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Dihydrosphingosine
Catalog No.:BCC6778
CAS No.:3102-56-5
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- Benzoquinonium dibromide
Catalog No.:BCC6641
CAS No.:311-09-1
- Taxifolin 3'-O-glucoside
Catalog No.:BCN6808
CAS No.:31106-05-5
- 3-Methyl-4-nitrobenzoic acid
Catalog No.:BCN2261
CAS No.:3113-71-1
- RFRP 3 (human)
Catalog No.:BCC6261
CAS No.:311309-27-0
- SYM 2081
Catalog No.:BCC6840
CAS No.:31137-74-3
- D-Xylose
Catalog No.:BCC8320
CAS No.:31178-70-8
- Sudan II
Catalog No.:BCN8383
CAS No.:3118-97-6
- Methylionene
Catalog No.:BCN7120
CAS No.:31197-54-3
- H-D-Ser-OH
Catalog No.:BCC2676
CAS No.:312-84-5
- Sideroxylin
Catalog No.:BCN5226
CAS No.:3122-87-0
- Eucalyptin
Catalog No.:BCN5227
CAS No.:3122-88-1
- Cimigenol-3-one
Catalog No.:BCN7430
CAS No.:31222-32-9
Involvement of heme oxygenase-1 induction in the cytoprotective and neuroinflammatory activities of Siegesbeckia Pubescens isolated from 5,3'-dihydroxy-3,7,4'-trimethoxyflavone in HT22 cells and BV2 cells.[Pubmed:27584055]
Int Immunopharmacol. 2016 Nov;40:65-72.
Glutamate-induced oxidative injury contributes to neuronal degeneration such as Alzheimer's disease, Parkinson's disease and Huntington's disease in the central nervous system (CNS). Siegesbeckia pubescens is used in oriental medicine to treat arthritis, stroke, rash, edema, and skin itching eczema in South-East Asia. This study provides evidence that 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (DTMF), a compound isolated from 90% MeOH extract of Siegesbeckia pubescens, effectively has neuroprotective and anti-neuroinflammatory activities. DTMF has cytoprotective and reactive oxygen species (ROS) reductive effects in HT22 cells. DTMF also decreased LPS-induced inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2) expression but attenuated LPS-induced nitrite (NO) and prostaglandin E2 (PGE2), as well as TNF-alpha and IL-1beta production. In addition, DTMF induced Heme oxygenase (HO)-1 expression, HO activity, nuclear transcription factor erythroid-2 related factor 2 (Nrf2) nuclear translocation, and antioxidant response element (ARE)-luciferase activity. DTMF increased p38 phosphorylation in HT22 cells and JNK phosphorylation in BV2 microglia cells. Thus, p38 inhibitor (SB203580) in HT22 cells and JNK inhibitor (SP600125) in BV2 microglia cells significantly suppressed HO-1 expression by DTMF. Furthermore, treatment with SnPP (an inhibitor of HO activity) significantly blocked the cytoprotective effects and the anti-neuroinflammatory action of DTMF. In addition, activated microglia-mediated cell death of mouse hippocampal HT22 cells was significantly repressed by DTMF. We also investigated the protective effect of DTMF on the death of glutamate-induced primary mouse hippocampal neurons. These results demonstrated that DTMF may be a good therapeutic agent against neurodegenerative diseases induced by oxidative stress.
3',5-dihydroxy-3,4',7-trimethoxyflavone-induces ER-stress-associated HCT-116 programmed cell death via redox signaling.[Pubmed:28103509]
Biomed Pharmacother. 2017 Apr;88:151-161.
Quercetin, a well cognized bioactive flavone possessing great medicinal value, has limited usage. The rapid gastrointestinal digestion of quercetin is also a major obstacle for its clinical implementation due to low bioavailability and poor aqueous solubility. 3',5-dihydroxy-3,4',7-trimethoxyflavone (DTMF), a novel semi-synthetic derivative of quercetin, is known to modulate several biological activities. Therefore, in the present study we examined the cytotoxic mechanism of DTMF in concentration-dependent manner (25, 50, and 100muM; 24h) against HCT-116 human colon carcinoma cells. The cytotoxic potential of DTMF was characterized based on deformed cell morphology, increased ROS accumulation, loss of mitochondrial membrane potential (Deltam), increased mitochondrial mass, chromatin condensation, and typical DNA-fragmentation in HCT-116 cells. The results showed that DTMF-induced enhanced ROS production at higher concentration (100muM) as evidenced by upregulated expression of ER stress and apoptotic proteins with concomitant increase in PERK, CHOP, and JNK levels, when compared to N-acetyl cysteine (NAC, ROS inhibitor) treated HCT-116 cells, which depicts that DTMF might act as a crucial mediator of apoptosis signaling. Collectively, our results suggest that DTMF stimulates ROS-mediated oxidative stress, which in turn induces PERK-CHOP and JNK pathway of apoptosis to promote HCT-116 cell death.
Diuretic effect of extracts, fractions and two compounds 2alpha,3beta,19alpha-trihydroxy-urs-12-en-28-oic acid and 5-hydroxy-3,6,7,8,4'-pentamethoxyflavone from Rubus rosaefolius Sm. (Rosaceae) leaves in rats.[Pubmed:28013356]
Naunyn Schmiedebergs Arch Pharmacol. 2017 Apr;390(4):351-360.
Although diuretics have been widely used to treat hypertension along with others cardiovascular and renal disorders, no scientific data have been recorded to support the diuretic properties of Rubus rosaefolius Sm. (Rosaceae), a plant popularly used in Brazil to treat hypertension. Male Wistar rats were orally treated with: vehicle; hydrochlorothiazide; aqueous (AERR) and methanolic (MERR) extracts; dichloromethane (DCM), hexane (HEX) and ethyl acetate (EA) fractions; and the isolated compounds 2alpha,3beta,19alpha-trihydroxy-urs-12-en-28-oic acid (TUA) and 5-hydroxy-3,6,7,8,4'-pentamethoxyflavone (PMF). At the end of the experiment (after 8 or 24 h), urine volume and other urine or plasma parameters were measured. AERR and MERR, at 100 and 30 mg/kg, respectively, induced diuretic, natriuretic and kaliuretic effect. Additionally, the DCM and HEX, but not EA, at 10 mg/kg, also increased urine volume and Na(+) and K(+) excretion. Both active constituents, TUA and PMF, at doses of 1 and 3 mg/kg, showed an augmented diuretic and natriuretic index. While TUA revealed a kaliuretic action, PMF did not interfere with potassium excretion. The compounds increased urinary creatinine, but not urea, levels. TUA was able to decrease calcium excretion, as well as HCTZ, while PMF effect was associated with increased urinary prostaglandin E2 levels. The non-selective muscarinic receptor antagonist (atropine) prevented TUA-induced diuresis. In addition, indomethacin (a cyclooxygenase inhibitor) and atropine, exhibited the ability to block the diuretic effects prompted by PMF. Our study demonstrates the diuretic effect of extracts, fractions and two natural compounds obtained from R. rosaefolius leaves in rats.
Sensitivity of lake sturgeon (Acipenser fulvescens) early life stages to 2,3,7,8-tetrachlorodibenzo-P-dioxin and 3,3',4,4',5-pentachlorobiphenyl.[Pubmed:27600767]
Environ Toxicol Chem. 2017 Apr;36(4):988-998.
The aquatic food web of the Great Lakes has been contaminated with polychlorinated biphenyls (PCBs) since the mid-20th century. Threats of PCB exposures to long-lived species of fish, such as lake sturgeon (Acipenser fulvescens), have been uncertain because of a lack of information on the relative sensitivity of the species. The objective of the present study was to evaluate the sensitivity of early-life stage lake sturgeon to 3,3',4,4',5-pentachlorobiphenyl (PCB-126) or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure. Mortality, growth, morphological and tissue pathologies, swimming performance, and activity levels were used as assessment endpoints. Pericardial and yolk sac edema, tubular heart, yolk sac hemorrhaging, and small size were the most commonly observed pathologies in both TCDD and PCB-126 exposures, beginning as early as 4 d postfertilization, with many of these pathologies occurring in a dose-dependent manner. Median lethal doses for PCB-126 and TCDD in lake sturgeon were 5.4 ng/g egg (95% confidence interval, 3.9-7.4 ng/g egg) and 0.61 ng/g egg (0.47-0.82 ng/g egg), respectively. The resulting relative potency factor for PCB-126 (0.11) was greater than the World Health Organization estimate for fish (toxic equivalency factor = 0.005), suggesting that current risk assessments may underestimate PCB toxicity toward lake sturgeon. Swimming activity and endurance were reduced in lake sturgeon survivors from the median lethal doses at 60 d postfertilization. Threshold and median toxicity values indicate that lake sturgeon, like other Acipenser species, are more sensitive to PCB and TCDD than the other genus of sturgeon, Scaphirhynchus, found in North America. Indeed, lake sturgeon populations in the Great Lakes and elsewhere are susceptible to PCB/TCDD-induced developmental toxicity in embryos and reductions in swimming performance. Environ Toxicol Chem 2017;36:988-998. Published 2016 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.


