Spaglumic acidPutative endogenous neurotransmitter CAS# 3106-85-2 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3106-85-2 | SDF | Download SDF |
| PubChem ID | 188803 | Appearance | Powder |
| Formula | C11H16N2O8 | M.Wt | 304.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | α-NAAG | ||
| Solubility | Soluble to 100 mM in water | ||
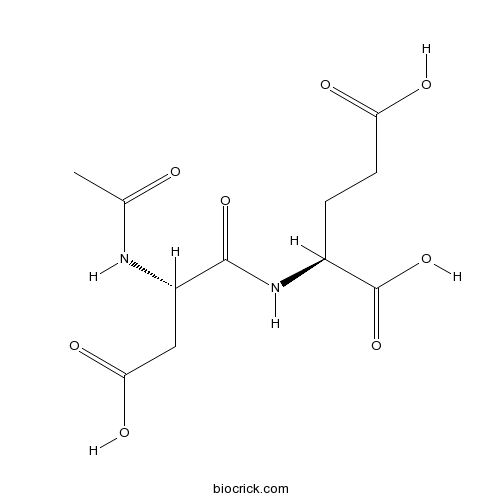
| Chemical Name | (2S)-2-[[(2S)-2-acetamido-3-carboxypropanoyl]amino]pentanedioic acid | ||
| SMILES | CC(=O)NC(CC(=O)O)C(=O)NC(CCC(=O)O)C(=O)O | ||
| Standard InChIKey | OPVPGKGADVGKTG-BQBZGAKWSA-N | ||
| Standard InChI | InChI=1S/C11H16N2O8/c1-5(14)12-7(4-9(17)18)10(19)13-6(11(20)21)2-3-8(15)16/h6-7H,2-4H2,1H3,(H,12,14)(H,13,19)(H,15,16)(H,17,18)(H,20,21)/t6-,7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The most abundant peptide neurotransmitter in the mammalian CNS. A weak activator of NMDA receptors, and a highly selective agonist for mGlu3 receptors. Neuroprotective under non-hydrolysing conditions in vivo. |

Spaglumic acid Dilution Calculator

Spaglumic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2867 mL | 16.4333 mL | 32.8666 mL | 65.7333 mL | 82.1666 mL |
| 5 mM | 0.6573 mL | 3.2867 mL | 6.5733 mL | 13.1467 mL | 16.4333 mL |
| 10 mM | 0.3287 mL | 1.6433 mL | 3.2867 mL | 6.5733 mL | 8.2167 mL |
| 50 mM | 0.0657 mL | 0.3287 mL | 0.6573 mL | 1.3147 mL | 1.6433 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3287 mL | 0.6573 mL | 0.8217 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Dihydrosphingosine
Catalog No.:BCC6778
CAS No.:3102-56-5
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Cedeodarin
Catalog No.:BCN4784
CAS No.:31076-39-8
- Alytesin
Catalog No.:BCC7203
CAS No.:31078-12-3
- Decylic acid vanillylamide
Catalog No.:BCN7836
CAS No.:31078-36-1
- PRT 4165
Catalog No.:BCC6354
CAS No.:31083-55-3
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone
Catalog No.:BCN1461
CAS No.:310888-07-4
- Benzoquinonium dibromide
Catalog No.:BCC6641
CAS No.:311-09-1
- Taxifolin 3'-O-glucoside
Catalog No.:BCN6808
CAS No.:31106-05-5
- 3-Methyl-4-nitrobenzoic acid
Catalog No.:BCN2261
CAS No.:3113-71-1
- RFRP 3 (human)
Catalog No.:BCC6261
CAS No.:311309-27-0
- SYM 2081
Catalog No.:BCC6840
CAS No.:31137-74-3
- D-Xylose
Catalog No.:BCC8320
CAS No.:31178-70-8
- Sudan II
Catalog No.:BCN8383
CAS No.:3118-97-6
Lodoxamide versus spaglumic acid: a comparative double-blind trial on patients suffering from seasonal allergic conjunctivitis induced by Parietaria pollen.[Pubmed:9395007]
Allergol Immunopathol (Madr). 1997 Sep-Oct;25(5):233-7.
In order to evaluate the efficacy and tolerability of lodoxamide in the treatment of allergic conjunctivitis, the authors conducted a double-blind trial with intrapatient comparison on 32 patients, using lodoxamide versus Spaglumic acid in the course of two conjunctival provocation tests performed with specific allergens. The patients received one drop of lodoxamide in one eye and one drop of Spaglumic acid in the other; 15 minutes later, 25 microliters of allergen extract at a pre-established concentration was instilled. After 10 minutes, the signs and symptoms of the allergic response were evaluated and recorded. Six hours later, the instillation of the allergen extract in both eyes was repeated following the same procedure, to establish the duration of the effect of the two drugs. The results, obtained by evaluating the main clinical signs and symptoms (itching, lacrimation, hyperaemia, palpebral oedema and chemosis), demonstrate with statistically significant differences that lodoxamide inhibits the conjunctival response to exposure to the allergen with greater efficacy than Spaglumic acid, and for a longer duration. The two drugs provided similar and satisfactory tolerability. In view of these results, lodoxamide can definitely be considered and effective drug in the treatment of allergic conjunctivitis.
N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system.[Pubmed:10899918]
J Neurochem. 2000 Aug;75(2):443-52.
In the progress of science, as in life, timing is important. The acidic dipeptide, N-acetylaspartylglutamate (NAAG), was discovered in the mammalian nervous system in 1965, but initially was not considered to be a neurotransmitter candidate. In the mid-1980s, a few laboratories revisited the question of NAAG's role in the nervous system and pursued hypotheses regarding its function that ranged from a precursor for the transmitter pool of glutamate to a direct role as a peptide transmitter. Since that time, NAAG has been tested against nearly all of the established criteria for identification of a neurotransmitter. It successfully meets each of these tests, including a concentrated presence in neurons and synaptic vesicles, release from axon endings in a calcium-dependent manner following initiation of action potentials, and extracellular hydrolysis by membrane-bound peptidase activity. NAAG is the most prevalent and widely distributed neuropeptide in the mammalian nervous system. NAAG activates NMDA receptors with a low potency that may vary among receptor subtypes, and it is a highly selective agonist at the type 3 metabotropic glutamate receptor (mGluR3). Acting through this receptor, NAAG reduces cyclic AMP levels, decreases voltage-dependent calcium conductance, suppresses excitotoxicity, influences long-term potentiation and depression, regulates GABA(A) receptor subunit expression, and inhibits synaptic release of GABA from cortical neurons. Cloning of peptidase activities against NAAG provides opportunities to study the cellular and molecular mechanisms by which synaptic NAAG peptidase activity is controlled. Given the codistribution of this peptide with a spectrum of traditional transmitters and its ability to activate mGluR3, we speculate that one role for NAAG following synaptic release is the activation of metabotropic autoreceptors that inhibit subsequent transmitter release. A second role is the production of extracellular glutamate following NAAG hydrolysis.
N-Acetylated alpha-linked acidic dipeptidase converts N-acetylaspartylglutamate from a neuroprotectant to a neurotoxin.[Pubmed:10991955]
J Pharmacol Exp Ther. 2000 Oct;295(1):16-22.
We previously reported that inhibition of the brain enzyme N-acetylated alpha-linked acidic dipeptidase (NAALADase; glutamate carboxypeptidase II) robustly protects cortical neurons from ischemic injury. Since NAALADase hydrolyzes N-acetylaspartylglutamate (NAAG) to glutamate we hypothesized that inhibiting NAALADase would both decrease glutamate and increase NAAG. Increasing NAAG is potentially important because NAAG is a metabotropic glutamate receptor agonist and an N-methyl-D-aspartate (NMDA) partial antagonist, both of which have previously been shown to be neuroprotective. To understand the likely effects of endogenous NAAG in the central nervous system, we have now investigated the activity of NAAG in primary cortical cultures while manipulating NAALADase activity. Under hydrolyzing conditions, when NAALADase was active, NAAG had toxic effects that were blocked by NMDA and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonists and by NAALADase inhibition. NAAG's toxic effects were presumably due to the liberation of glutamate. Under nonhydrolyzing conditions, when NAALADase was inhibited, NAAG demonstrated neuroprotective effects against both NMDA toxicity and metabolic inhibition. In the case of NMDA-induced toxicity, NAAG provided neuroprotection through its partial antagonist activity at the NMDA receptor. In the case of metabolic inhibition, NAAG had an additional neuroprotective effect mediated through its agonist properties at the type II metabotropic glutamate receptor. These results indicate that NAAG might play an important role in the central nervous system, under certain pathological conditions, as a neurotoxin or as a neuroprotectant, depending on the activity of NAALADase.
N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells.[Pubmed:9202308]
J Neurochem. 1997 Jul;69(1):174-81.
In previous studies, we demonstrated that the neuropeptide, N-acetylaspartylglutamate (NAAG), meets the traditional criteria for a neurotransmitter and selectively activates metabotropic glutamate receptor mGluR2 or mGluR3 in cultured cerebellar granule cells and glia. Sequence homology and pharmacological data suggest that these two receptors are highly related structurally and functionally. To define more rigorously the receptor specificity of NAAG, cloned rat cDNAs for mGluR1-6 were transiently or stably transfected into Chinese hamster ovary cells and human embryonic kidney cells and assayed for their second messenger responses to the two endogenous neurotransmitters, glutamate and NAAG, as well as to metabotropic receptor agonists, trans-1-aminocyclopentane-1,3-dicarboxylate (trans-ACPD) and L-2-amino-4-phosphonobutyrate (L-AP4). Despite the high degree of relatedness of mGluR2 and mGluR3, NAAG selectively activated the mGluR3 receptor. NAAG activated neither mGluR2 nor mGluR1, mGluR4, mGluR5, or mGluR6. The mGluR agonist, trans-ACPD, activated each of the transfected receptors, whereas L-AP4 activated mGluR4 and mGluR6, consistent with the published selectivity of these agonists. Hybrid cDNA constructs of the extracellular domains of mGluR2 and mGluR3 were independently fused with the transmembrane and cytoplasmic domain of mGluR1a. This latter receptor domain is coupled to phosphoinositol turnover, and its activation increases intracellular calcium. The cells transfected with these chimeric receptors responded to activation by glutamate and trans-ACPD with increases in intracellular calcium. NAAG activated the chimeric receptor that contained the extracellular domain of mGluR3 and did not activate the mGluR2 chimera.


