4-HQNPARP inhibitor CAS# 491-36-1 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 491-36-1 | SDF | Download SDF |
| PubChem ID | 63112 | Appearance | Powder |
| Formula | C8H6N2O | M.Wt | 146.15 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 100 mM in DMSO | ||
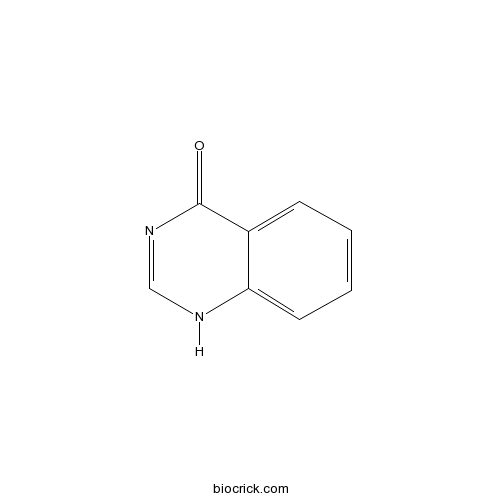
| Chemical Name | 1H-quinazolin-4-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)N=CN2 | ||
| Standard InChIKey | QMNUDYFKZYBWQX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H6N2O/c11-8-6-3-1-2-4-7(6)9-5-10-8/h1-5H,(H,9,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Some 4-quinazolinone derivatives have antiplatelet activity. |

4-HQN Dilution Calculator

4-HQN Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.8423 mL | 34.2114 mL | 68.4229 mL | 136.8457 mL | 171.0571 mL |
| 5 mM | 1.3685 mL | 6.8423 mL | 13.6846 mL | 27.3691 mL | 34.2114 mL |
| 10 mM | 0.6842 mL | 3.4211 mL | 6.8423 mL | 13.6846 mL | 17.1057 mL |
| 50 mM | 0.1368 mL | 0.6842 mL | 1.3685 mL | 2.7369 mL | 3.4211 mL |
| 100 mM | 0.0684 mL | 0.3421 mL | 0.6842 mL | 1.3685 mL | 1.7106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Inhibitor of poly(ADP-ribose) polymerase (PARP) (IC50 = 9.5 μM); displays mixed inhibition with respect to NAD+. Protective against ischemia-reperfusion induced ROS production, and subsequent mitochondrial and cell damage in rat heart. Anti-inflammatory in LPS-induced endotoxic mice in vivo; decreases NF-κB and AP-1 activation.
- Valeroidine
Catalog No.:BCN1920
CAS No.:490-96-0
- Gentisic acid
Catalog No.:BCN3408
CAS No.:490-79-9
- 2,5-Dihydroxyacetophenone
Catalog No.:BCN3825
CAS No.:490-78-8
- Homoquinolinic acid
Catalog No.:BCC6570
CAS No.:490-75-5
- Gaultherin
Catalog No.:BCN2482
CAS No.:490-67-5
- Epicatechin
Catalog No.:BCN5597
CAS No.:490-46-0
- Robinetin
Catalog No.:BCN5596
CAS No.:490-31-3
- Beta-Tocotrienol
Catalog No.:BCN3725
CAS No.:490-23-3
- a-Truxilline
Catalog No.:BCN1947
CAS No.:490-17-5
- SBC-115076
Catalog No.:BCC6440
CAS No.:489415-96-5
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- Quercetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN1261
CAS No.:491-50-9
- Kaempferide
Catalog No.:BCN5598
CAS No.:491-54-3
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Isosakuranin
Catalog No.:BCN3712
CAS No.:491-69-0
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Chrysoeriol
Catalog No.:BCN5601
CAS No.:491-71-4
- Iridin
Catalog No.:BCN6868
CAS No.:491-74-7
- Biochanin A
Catalog No.:BCN1224
CAS No.:491-80-5
- Hemokinin 1 (human)
Catalog No.:BCC5923
CAS No.:491851-53-7
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
- Thermopsidine
Catalog No.:BCN7923
CAS No.:492-02-4
- (+)-Sparteine
Catalog No.:BCC9249
CAS No.:492-08-0
Synthesis and in vitro study of platelet antiaggregant activity of some 4-quinazolinone derivatives.[Pubmed:1635928]
Pharmazie. 1992 Feb;47(2):91-4.
Some new 4-quinazolinones were prepared. Their antiplatelet activity was evaluated in vitro with respect to aggregation induced by ADP, collagen, arachidonic acid and the platelet serotonin release reaction. Most molecules showed an inhibiting power similar to that of acetylsalicylic acid under the same conditions, and even greater when aggregation was induced by ADP. Reduction of the 4-quinazolinone derivatives to their 1,2,3,4-tetrahydroquinazoline homologues produced an increase in platelet inhibitory action except when ADP is the inductor.
Regulation of kinase cascades and transcription factors by a poly(ADP-ribose) polymerase-1 inhibitor, 4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice.[Pubmed:14999056]
J Pharmacol Exp Ther. 2004 Jul;310(1):247-55.
Activation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP) is involved in numerous pathophysiological conditions. Because PARP-1 knockout mice are resistant to endotoxin-induced shock and inhibitors of the enzyme were reported to have similar beneficial properties, we investigated the effect of 4-hydroxyquinazoline (4-HQN), a potent PARP-1 inhibitor, on the modulation of kinase cascades and the regulation of transcription factors in a rodent septic shock model. T2-weighted magnetic resonance imaging showed the pattern of anatomical localization of the inflammatory response in bacterial lipopolysaccharide (LPS)-treated mice and the anti-inflammatory effect of the PARP-1 inhibitor. We have found that 4-HQN activated the phosphatidylinositol 3 (PI3)-kinase/Akt pathway in lung, liver, and spleen, and down-regulated two elements of the MAP kinase system. Namely, it dramatically attenuated the activation of the LPS-induced extracellular signal-regulated kinase (ERK)1/2 and p38 mitogen-activated protein (MAP) kinase in a tissue-specific manner. Furthermore, phosphorylation of p90RSK, a downstream target of ERK1/2, showed a similar pattern of down-regulation as did the phosphorylation of ERK1/2 and p38 after LPS and 4-HQN treatment. As a consequence of the aforementioned effects on the kinase pathways, 4-HQN decreased the activation of transcription factor nuclear factor-kappaB (NF-kappaB) and activator protein 1 (AP-1) in LPS-induced endotoxic shock. Our results provide evidence for the first time that the beneficial effects of PARP inhibition in endotoxic shock, such as attenuation of NF-kappaB- and AP-1 transcription factor activation, are mediated, at least partially, through the regulation of the PI3-kinase/Akt pathway and MAP kinase cascades.
Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system.[Pubmed:11353811]
Mol Pharmacol. 2001 Jun;59(6):1497-505.
Ischemia-reperfusion induces reactive oxygen species (ROS) formation, and ROS lead to cardiac dysfunction, in part, via the activation of the nuclear poly(ADP-ribose) polymerase (PARP, called also PARS and ADP-RT). ROS and peroxynitrite induce single-strand DNA break formation and PARP activation, resulting in NAD(+) and ATP depletion, which can lead to cell death. Although protection of cardiac muscle by PARP inhibitors can be explained by their attenuating effect on NAD(+) and ATP depletion, there are data indicating that PARP inhibitors also protect mitochondria from oxidant-induced injury. Studying cardiac energy metabolism in Langendorff heart perfusion system by (31)P NMR, we found that PARP inhibitors (3-aminobenzamide, nicotinamide, BGP-15, and 4-hydroxyquinazoline) improved the recovery of high-energy phosphates (ATP, creatine phosphate) and accelerated the reutilization of inorganic phosphate formed during the ischemic period, showing that PARP inhibitors facilitate the faster and more complete recovery of the energy production. Furthermore, PARP inhibitors significantly decrease the ischemia-reperfusion-induced increase of lipid peroxidation, protein oxidation, single-strand DNA breaks, and the inactivation of respiratory complexes, which indicate a decreased mitochondrial ROS production in the reperfusion period. Surprisingly, PARP inhibitors, but not the chemically similar 3-aminobenzoic acid, prevented the H(2)O(2)-induced inactivation of cytochrome oxidase in isolated heart mitochondria, suggesting the presence of an additional mitochondrial target for PARP inhibitors. Therefore, PARP inhibitors, in addition to their important primary effect of decreasing the activity of nuclear PARP and decreasing NAD(+) and ATP consumption, reduce ischemia-reperfusion-induced endogenous ROS production and protect the respiratory complexes from ROS induced inactivation, providing an additional mechanism by which they can protect heart from oxidative damages.
Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferase.[Pubmed:1530940]
J Biol Chem. 1992 Jan 25;267(3):1569-75.
Two classes of enzymes, poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferases, catalyze covalent attachment of multiple or single residues, respectively, of the ADP-ribose moiety of NAD+ to various proteins. In order to find good inhibitors of poly(ADP-ribose) synthetase free of side actions and applicable to in vivo studies, we made a large scale survey using an in vitro assay system, and found many potent inhibitors. The four strongest were 4-amino-1,8-naphthalimide, 6(5H)- and 2-nitro-6(5H)-phenanthridinones, and 1,5-dihydroxyisoquinoline. Their 50% inhibitory concentrations, 0.18-0.39 microM, were about two orders of magnitude lower than that of 3-aminobenzamide that is currently most popularly used. A common structural feature among all potent inhibitors, including 1-hydroxyisoquinoline, chlorthenoxazin, 3-hydroxybenzamide, and 4-hydroxyquinazoline, in addition to the four mentioned above, was the presence of a carbonyl group built in a polyaromatic heterocyclic skeleton or a carbamoyl group attached to an aromatic ring. Most of the inhibitors exhibited mixed-type inhibition with respect to NAD+. Comparative studies of the effects on poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferase from hen heterophils revealed high specificity of most of the potent inhibitors for poly(ADP-ribose) synthetase. On the other hand, unsaturated long-chain fatty acids inhibited both enzymes, and saturated long-chain fatty acids and vitamin K1 acted selectively on mono(ADP-ribosyl)transferase. The finding of many inhibitors of ADP-ribosyltransferases, especially poly(ADP-ribose) synthetase, supports the view that ADP-ribosylation of proteins may be regulated by a variety of metabolites or structural constituents in the cell.


