10-hydroxydec-2-enoic acidCAS# 14113-05-4 |
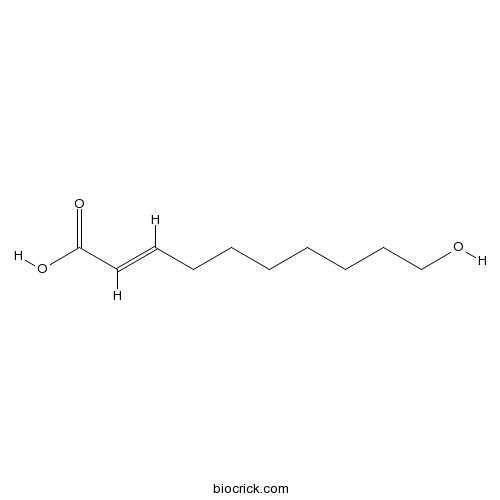
- 10-Hydroxy-2-decenoic acid
Catalog No.:BCN2654
CAS No.:765-01-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14113-05-4 | SDF | Download SDF |
| PubChem ID | 5312738 | Appearance | White powder |
| Formula | C10H18O3 | M.Wt | 186.25 |
| Type of Compound | Aliphatic Compounds | Storage | Desiccate at -20°C |
| Synonyms | 10H2DA | ||
| Solubility | Soluble in ethanol and methanol; insoluble in water | ||
| Chemical Name | (E)-10-hydroxydec-2-enoic acid | ||
| SMILES | C(CCCC=CC(=O)O)CCCO | ||
| Standard InChIKey | QHBZHVUGQROELI-SOFGYWHQSA-N | ||
| Standard InChI | InChI=1S/C10H18O3/c11-9-7-5-3-1-2-4-6-8-10(12)13/h6,8,11H,1-5,7,9H2,(H,12,13)/b8-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

10-hydroxydec-2-enoic acid Dilution Calculator

10-hydroxydec-2-enoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3691 mL | 26.8456 mL | 53.6913 mL | 107.3826 mL | 134.2282 mL |
| 5 mM | 1.0738 mL | 5.3691 mL | 10.7383 mL | 21.4765 mL | 26.8456 mL |
| 10 mM | 0.5369 mL | 2.6846 mL | 5.3691 mL | 10.7383 mL | 13.4228 mL |
| 50 mM | 0.1074 mL | 0.5369 mL | 1.0738 mL | 2.1477 mL | 2.6846 mL |
| 100 mM | 0.0537 mL | 0.2685 mL | 0.5369 mL | 1.0738 mL | 1.3423 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Citral
Catalog No.:BCN9066
CAS No.:5392-40-5
- Eugenol acetate
Catalog No.:BCN9065
CAS No.:93-28-7
- Alizarin 1-methyl ether
Catalog No.:BCN9064
CAS No.: 6170-06-5
- D-Ribose
Catalog No.:BCN9063
CAS No.:50-69-1
- α-L-Rhamnopyranose
Catalog No.:BCN9062
CAS No.:6014-42-2
- (±)-Naringenin
Catalog No.:BCN9061
CAS No.:67604-48-2
- Glucodigifucoside
Catalog No.:BCN9060
CAS No.:2446-63-1
- Trimethyl phosphate
Catalog No.:BCN9059
CAS No.:512-56-1
- N-Phenethylbenzamide
Catalog No.:BCN9058
CAS No.:3278-14-6
- Warfarin sodium
Catalog No.:BCN9057
CAS No.:129-06-6
- Flavokawain A
Catalog No.:BCN9056
CAS No.:37951-13-6
- 5'-Guanylic acid
Catalog No.:BCN9055
CAS No.:85-32-5
- Citronellal
Catalog No.:BCN9068
CAS No.:106-23-0
- Benzyl alcohol
Catalog No.:BCN9069
CAS No.:100-51-6
- (-)-Menthone
Catalog No.:BCN9070
CAS No.:14073-97-3
- (+)-Fenchone
Catalog No.:BCN9071
CAS No.:4695-62-9
- Alphalipoic acid
Catalog No.:BCN9072
CAS No.:1077-28-7
- Ferulic Acid Methyl Ester
Catalog No.:BCN9073
CAS No.:2309-07-1
- 3-(β-D-Glucopyranosyloxy)-1,6-dihydroxy-2-methyl-9,10-anthracenedione
Catalog No.:BCN9074
CAS No.:125906-49-2
- (-)-Fenchone
Catalog No.:BCN9075
CAS No.:7787-20-4
- (1S)-(-)-α-Pinene
Catalog No.:BCN9076
CAS No.:7785-26-4
- (S)-(+)-Carvone
Catalog No.:BCN9077
CAS No.:2244-16-8
- Sodium taurocholate
Catalog No.:BCN9078
CAS No.:145-42-6
- Sodium deoxycholate
Catalog No.:BCN9079
CAS No.:302-95-4
The key royal jelly component 10-hydroxy-2-decenoic acid protects against bone loss by inhibiting NF-kappaB signaling downstream of FFAR4.[Pubmed:32647011]
J Biol Chem. 2020 Jul 9. pii: RA120.013821.
The supplementation of royal jelly (RJ) is known to provide a variety of health benefits, including anti-inflammatory and anti-obesity effects. RJ treatment also reportedly protects against bone loss, but no single factor in RJ has yet been identified as an anti-osteoporosis agent. Here we fractionated RJ and identified 10-hydroxy-2-decenoic acid (10H2DA) as a key component involved in inhibiting osteoclastogenesis based on mass spectrometric analysis. We further demonstrated free fatty acid receptor 4 (FFAR4) as directly interacting with 10H2DA; binding of 10H2DA to FFAR4 on osteoclasts inhibited receptor activator of nuclear factor-kappaB (NF-kappaB) ligand (RANKL)-induced activation of NF-kappaB signaling, thereby attenuating the induction of nuclear factor of activated T cells (NFAT) c1, a key transcription factor for osteoclastogenesis. Oral administration of 10H2DA attenuated bone resorption in ovariectomized mice. These results suggest a potential therapeutic approach of targeting osteoclast differentiation by the supplementation of RJ, and specifically 10H2DA, in cases of pathological bone loss such as occur in postmenopausal osteoporosis.
Transcriptional regulator SpxA1a controls the resistance of the honey bee pathogen Melissococcus plutonius to the antimicrobial activity of royal jelly.[Pubmed:32519428]
Environ Microbiol. 2020 Jul;22(7):2736-2755.
Royal jelly (RJ), a brood food of honey bees, has strong antimicrobial activity. Melissococcus plutonius, the causative agent of European foulbrood of honey bees, exhibits resistance to this antimicrobial activity and infects larvae orally. Among three genetically distinct groups (CC3, CC12 and CC13) of M. plutonius, CC3 strains exhibit the strongest RJ resistance. In this study, to identify genes involved in RJ resistance, we generated an RJ-susceptible derivative from a highly RJ-resistant CC3 strain by UV mutagenesis. Genome sequence analysis of the derivative revealed the presence of a frameshift mutation in the putative regulator gene spxA1a. The deletion of spxA1a from a CC3 strain resulted in increased susceptibility to RJ and its antimicrobial component 10-hydroxy-2-decenoic acid. Moreover, the mutant became susceptible to low-pH and oxidative stress, which may be encountered in brood foods. Differentially expressed gene analysis using wild-type and spxA1a mutants revealed that 45 protein-coding genes were commonly upregulated in spxA1a-positive strains. Many upregulated genes were located in a prophage region, and some highly upregulated genes were annotated as universal/general stress proteins, oxidoreductase/reductase, chaperons and superoxide dismutase. These results suggest that SpxA1a is a key regulator to control the tolerance status of M. plutonius against stress in honey bee colonies.
A Validated Stability-Indicating HPTLC Assay for Determination of 10-Hydroxy-2-Decenoic Acid Content in Royal Jelly Products Using Robust Regression Methods.[Pubmed:32390054]
J Chromatogr Sci. 2020 Jun 5;58(6):520-534.
A new, simple, stability-indicating high-performance thin-layer chromatography method was developed for the quantification of 10-hydroxy-2-decenoic acid (10-HDA) in some royal jelly products marketed in Egypt. The used solvent system was chloroform:acetic acid (10:1, v/v) and the bands were measured densitometrically at 210 nm. First- and second-derivative treatments of the data were performed. The present study shows a comparison between three statistical regression methods for handling data: parametric, nonparametric and weighted regression (WR) methods. The developed methods were validated as per International Conference on Harmonization guidelines. To validate the stability-indicating power of the developed analytical method, the royal jelly standard was subjected to forced degradation studies including the effect of hydrolysis, oxidation, photolysis and dry heat. It was found that derivative treatment of the chromatographic response data gives improved quantitation and sensitivity of the chromatographic signals. Weighted regression of the response data is found to be advantageous over the use of both parametric and nonparametric regression models. This was shown by a great enhancement in the accuracy and precision in the analysis of 10-HDA in royal jelly products. The % recovery in case of WR was 99.92 +/- 0.16, while % recovery in case of nonparametric and parametric regressions were 99.56 +/- 0.25 and 98.63 +/- 0.65, respectively.
10-Hydroxy-2-Decenoic Acid Prevents Ultraviolet A-Induced Expression of Lamin AA150 in Human Dermal Fibroblasts.[Pubmed:32153662]
Maedica (Buchar). 2019 Dec;14(4):327-331.
10-Hydroxy-2-decenoic acid (10-HDA) as the main component of royal jelly has pharmacological characteristics. But the influence of 10-HDA on skin photoaging and photo damage is poorly understood. In the present study, we used 10-HAD immediately after UVA exposure and tested the effects on the attenuation of LMNAA150 expression in cultured human dermal fibroblasts Human dermal fibroblasts (cultured cells) were exposed to UVA irradiation. The mRNA level of LMNAA150 was determined by Taqman Real-Time PCR Assay. Real-time PCR analysis of LMNAA150 transcripts indicated that the level of LMNAA150 transcripts was higher in the UVA exposed group than the group treated with 10-HAD after UVA exposure (>8.22-fold). The LMNAA150 expression is down-regulated in human dermal fibroblasts after treatment with 10-HDA. It can be concluded that treatment with 10-HDA suppresses the UVA-induced gene expression of LMNAA150 and protects skin from UVA-induced photoaging and photo damage.
Study of the Royal Jelly Free Fatty Acids by Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS).[Pubmed:31963373]
Metabolites. 2020 Jan 16;10(1). pii: metabo10010040.
The lipidome of royal jelly (RJ) consists of medium-chained (8-12 carbon atoms) free fatty acids. We present herein a liquid chromatography-high resolution mass spectrometry (HRMS) method that permits the determination of RJ fatty acids and at the same time the detection of suspect fatty acids. The method allows for the direct quantification of seven free fatty acids of RJ, avoiding any derivatization step. It was validated and applied in seven RJ samples, where the major RJ fatty acid trans-10-hydroxy-2-decenoic acid (10-HDA) was found to vary from 0.771 +/- 0.08 to 0.928 +/- 0.04 g/100 g fresh RJ. Four additional suspect fatty acids were simultaneously detected taking advantage of the HRMS detection.
Trans-10-hydroxy-2-decenoic acid protects against LPS-induced neuroinflammation through FOXO1-mediated activation of autophagy.[Pubmed:31820078]
Eur J Nutr. 2019 Dec 9. pii: 10.1007/s00394-019-02128-9.
PURPOSE: Neuroinflammation is thought to be associated with the pathogenesis of a series of neurodegenerative diseases. We have previously reported that royal jelly (RJ) has an anti-inflammatory effect on microglial BV-2 cells. However, components contributing to the effect of RJ were largely unexplored. The aim of this study was to assess whether trans-10-hydroxy-2-decenoic acid (10-HDA), the exclusive fatty acid in RJ, can alleviate neuroinflammation and to further explore the underlying mechanisms. METHODS: Immunohistochemistry staining, ELISA, qRT-PCR and Western blot were used to assess the effect of 10-HDA on LPS-induced neuroinflammation both in vivo and in vitro. To determine the extent of inflammatory changes after 10-HDA treatment, RNAseq transcriptomic analysis was conducted. RESULTS: 10-HDA pretreatment significantly reduced the production of pro-inflammatory mediators in LPS-treated C57BL/6J mice and microglial BV-2 cells. 10-HDA inhibited the activation of the TNF-alpha/NF-kappaB axis and NLRP3 inflammasome-IL-1beta pathway, which may be the anti-neuroinflammatory mechanism of 10-HDA. We also demonstrated that 10-HDA triggered cell autophagy, as evidenced by elevated levels of microtubule-associated protein 1 light chain 3-II (LC3-II) and decreased expression of SQSTM1. More importantly, 10-HDA increased the transcriptional activity of FOXO1 by increasing FOXO1 nuclear localization. Inhibition of FOXO1 and autophagy using chemical inhibitors markedly blunted the effect of 10-HDA on the TNF-alpha pathway and NLRP3 inflammasome-IL-1beta pathway, indicating that 10-HDA alleviates neuroinflammation in BV-2 cells by modulating FOXO1-mediated autophagy. CONCLUSIONS: 10-HDA may be a promising agent for various neuroinflammation-associated diseases.
Down-regulation of aquaporin 9 gene transcription by 10-hydroxy-2-decenoic acid: A major fatty acid in royal jelly.[Pubmed:31763031]
Food Sci Nutr. 2019 Oct 21;7(11):3819-3826.
10-Hydroxy-trans-2-decenoic acid (10H2DA) is a unique lipid component of royal jelly produced by worker honeybees that exerts insulin-like effects. We herein investigated the effects of 10H2DA on the gene expression of aquaporin 9 (AQP9), which functions as a glycerol transporter in the liver, to clarify whether 10H2DA modulates energy metabolism. 10H2DA suppressed AQP9 gene expression in HepG2 cells by promoting the phosphorylation of Akt and AMP-activated protein kinase (AMPK). This suppression was partially recovered by the treatment of cells with inhibitors for Akt and AMPK. Based on the result showing that leptomycin B partially recovered the suppression of AQP9 gene expression, 10H2DA inhibited the expression of Foxa2, a transcription factor for the AQP9 gene, and also induced its nuclear exclusion. Although 10H2DA up-regulated phosphoenolpyruvate carboxykinase and glucose-6-phosphatase gene expression, this was suppressed through the modulation of Foxa2 by insulin. These results suggest that 10H2DA suppresses AQP9 gene expression through the phosphorylation of Akt and AMPK and down-regulation of Foxa2 expression.
Trans-10-hydroxy-2-decenoic acid alleviates LPS-induced blood-brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway.[Pubmed:31614141]
Eur J Pharmacol. 2019 Dec 15;865:172736.
We previously reported that trans-10-hydroxy-2-decenoic acid (10-HDA), the exclusive lipid component of royal jelly (RJ), alleviates Lipopolysaccharide (LPS)-induced neuroinflammation both in vivo and in vitro. However, whether 10-HDA can protect against LPS-induced blood-brain barrier (BBB) damage is largely unexplored. In this study, we first observed that 10-HDA decreased BBB permeability in LPS-stimulated C57BL/6 mice by Evan's blue (EB) dye. Immunostaining and Western blot results showed that 10-HDA alleviated BBB dysfunction by inhibiting the degradation of tight junction proteins (occludin, claudin-5 and ZO-1). In LPS-stimulated human brain microvascular endothelial cells (HBMECs), 10-HDA decreased the expression of chemokines (CCL-2 and CCL-3), adhesion molecules (ICAM-1 and VCAM-1), reactive oxygen species, matrix metalloproteinases (MMP-2 and MMP-9) and increased the expression of tight junction proteins. Interestingly, LC-MS/MS analysis showed that 10-HDA pretreatment upregulated the expression of mitochondria-associated proteins, which may reflect the mechanism underlying the regulatory effect of 10-HDA on reactive oxygen species. We further illustrated that 10-HDA promoted the activation of the AMPK pathway and the downstream PI3K/AKT pathway. Compound C (an AMPK inhibitor) and LY294002 (a PI3K inhibitor) markedly reversed the alleviating effect of 10-HDA on the expression of tight junction proteins, indicating that 10-HDA inhibited LPS-induced BBB dysfunction by triggering the activation of the AMPK/PI3K/AKT pathway. Collectively, these data reveal that 10-HDA may be an interesting candidate for clinical evaluation in the treatment of diseases related to BBB damage.
Determination of 10-Hydroxy-2-Decenoic Acid of Royal Jelly Using Near-Infrared Spectroscopy Combined with Chemometrics.[Pubmed:31483872]
J Food Sci. 2019 Sep;84(9):2458-2466.
A rapid quantitative analysis model for determining the hydroxy-2-decenoic acid (10-HDA) content of royal jelly based on near-infrared spectroscopy combining with PLS has been developed. Firstly, near-infrared spectra of 232 royal jelly samples with different 10-HDA concentrations (0.35% to 2.44%) were be collected. Second-order derivative processing of the spectra was carried out to construct a full-spectrum PLS model. Secondly, GA-PLS, CARS-PLS, and Si-PLS were used to select characteristic wavelengths from the second-order derivative spectrum to construct a PLS calibration model. Finally, 58 samples were used to select the best predictive model for 10-HDA content. The result show that the PLS model constructed after wavelength selection was significantly more accurate than the full spectrum model. The Si-PLS algorithm performed best and the corresponding characteristic wavelength range were: 980 to 1038, 1220 to 1278, 1340 to 1398, and 1688 to 1746 nm. The prediction results were RMSEP = 0.1496% and RP = 0.9380. Hence, it is feasible to employ near-infrared spectra to analyze 10-HDA in royal jelly.
Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms.[Pubmed:30696450]
BMC Complement Altern Med. 2019 Jan 29;19(1):33.
BACKGROUND: Royal jelly (RJ) has been used traditionally for dietary, cosmetic and health purposes for a long time in different parts of the world. Scientific studies have also shown its numerous health-promoting properties including hypoglycemic and anti-hypercholesterolemic action. In this study, we investigated the anti-adipogenic activity of RJ in 3 T3-L1 cells and isolated the major responsible root component for the activity. METHODS: An active anti-adipogenic compound was isolated through bioassay-guided isolation process by successive treatment of RJ and its active fractions on 3 T3-L1 cell line. (E)-10-Hydroxy-2-decenoic Acid (10-HDA) was identified using NMR spectroscopy and ultra-performance liquid chromatography (UPLC). As 10-HDA showed significant anti-adipogenic activity with Oil Red O staining and TG content assay on 3 T3-L1 adipocytes, further study was carried out in molecular level for the expression of adipogenic transcription factors such as PPARgamma, FABP4, C/EBPalpha, SREBP-1c, and Leptin. The effect of 10-HDA on preliminary molecules such as pAkt, pERK, C/EBPbeta, and pCREB were studied in the early stage of adipogenesis. The effect of 10-HDA on reactive oxygen species (ROS) production in fully differentiating adipocytes was measured by nitro blue tetrazolium (NBT) assay. RESULT: Results showed that triacylglycerol accumulation and ROS production was markedly suppressed by 10-HDA. Preliminary molecules such as pAkt, pERK, pCERB, and C/EBPbeta were found to be down-regulated by 10-HDA, which led to down-regulation of key adipogenic transcription factors such as PPARgamma, FABP4, CEBPalpha, SREBP-1c, and Leptin on 3 T3-L1 adipocytes. CONCLUSION: Our results suggest that anti-adipogenesis of 10-HDA on 3 T3-L1 adipocyte takes place via two mechanisms: inhibition of cAMP/PKA pathway and inhibition of p-Akt and MAPK dependent insulin signaling pathway. So it is considered that 10-HDA, a major component of RJ, can be a potential therapeutic medicine for obesity.
10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae.[Pubmed:30544571]
Molecules. 2018 Dec 7;23(12). pii: molecules23123236.
Paenibacillus larvae (P. larvae) is a bacterial pathogen causing American foulbrood (AFB), the most serious disease of honeybee larvae. The food of young larvae could play an important role in the resistance of larvae against AFB. It contains antibacterial substances produced by honeybees that may inhibit the propagation of the pathogen in larval midguts. In this study, we identified and investigated the antibacterial effects of one of these substances, trans-10-hydroxy-2-decenoic acid (10-HDA), against P. larvae strains including all Enterobacterial Repetitive Intergenic Consensus (ERIC) genotypes. Its inhibitory activities were studied by determining the minimum inhibitory concentrations (MICs). It was found that 10-HDA efficacy increases substantially with decreasing pH; up to 12-fold differences in efficacy were observed between pH = 5.5 and pH = 7.2. P. larvae strains showed different susceptibility to 10-HDA; up to 2.97-fold differences existed among various strains with environmentally important ERIC I and ERIC II genotypes. Germinating spores of the pathogen were generally more susceptible to 10-HDA than vegetative cells. Our findings suggest that 10-HDA could play significant role in conferring antipathogenic activity to larval food in the midguts of young larvae and contribute to the resistance of individual larvae to P. larvae.
Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies.[Pubmed:30347885]
Int J Mol Sci. 2018 Oct 21;19(10). pii: ijms19103270.
Royal jelly (RJ) is a glandular secretion produced by worker honeybees and is a special food for the queen honeybee. It results in a significant prolongation of the lifespan of the queen honeybee compared with the worker honeybees through anti-inflammatory, anti-oxidant and anti-microbial activities. Consequently, RJ is used as cosmetic and dietary supplement throughout the world. In addition, in vitro studies and animal experiments have demonstrated that RJ inhibits cell proliferation and stimulates apoptosis in various types of malignant cells and affects the production of various chemokines, anti-oxidants and growth factors and the expression of cancer-related molecules in patients with malignancies, especially in patients treated with anti-cancer agents. Therefore, RJ is thought to exert anti-cancer effects on tumor growth and exhibit protective functions against drug-induced toxicities. RJ has also been demonstrated to be useful for suppression of adverse events, the maintenance of the quality of life during treatment and the improvement of prognosis in animal models and patients with malignancies. To understand the mechanisms of the beneficial effects of RJ, knowledge of the changes induced at the molecular level by RJ with respect to cell survival, inflammation, oxidative stress and other cancer-related factors is essential. In addition, the effects of combination therapies of RJ and other anti-cancer agents or natural compounds are important to determine the future direction of RJ-based treatment strategies. Therefore, in this review, we have covered the following five issues: (1) the anti-cancer effects of RJ and its main component, 10-hydroxy-2-decenoic acid; (2) the protective effects of RJ against anti-cancer agent-induced toxicities; (3) the molecular mechanisms of such beneficial effects of RJ; (4) the safety and toxicity of RJ; and (5) the future directions of RJ-based treatment strategies, with a discussion on the limitations of the study of the biological activities of RJ.
Induction of Human-Lung-Cancer-A549-Cell Apoptosis by 4-Hydroperoxy-2-decenoic Acid Ethyl Ester through Intracellular ROS Accumulation and the Induction of Proapoptotic CHOP Expression.[Pubmed:30296076]
J Agric Food Chem. 2018 Oct 17;66(41):10741-10747.
Royal jelly, a natural product secreted by honeybees, contains several fatty acids, such as 10-hydroxy-2-decenoic acid (DE), and shows anti- and pro-apoptotic properties. 4-Hydroperoxy-2-decenoic acid ethyl ester (HPO-DAEE), a DE derivative, exhibits potent antioxidative activity; however, it currently remains unclear whether HPO-DAEE induces cancer-cell death. In the present study, treatment with HPO-DAEE induced human-lung-cancer-A549-cell death (52.7 +/- 10.2%) that was accompanied by DNA fragmentation. Moreover, the accumulation of intracellular reactive oxygen species (ROS, 2.38 +/- 0.1-fold) and the induction of proapoptotic CCAAT-enhancer-binding-protein-homologous-protein (CHOP) expression (18.4 +/- 4.0-fold) were observed in HPO-DAEE-treated cells. HPO-DAEE-elicited CHOP expression and cell death were markedly suppressed by pretreatment with N-acetylcysteine (NAC), an antioxidant, by 2.40 +/- 1.57-fold and 5.7 +/- 1.6%, respectively. Pretreatment with 4-phenylbutyric acid (PBA), an inhibitor of endoplasmic reticulum stress, also suppressed A549-cell death (38.4 +/- 1.1%). Furthermore, we demonstrated the involvement of extracellular-signal-regulated protein kinase (ERK) and p38-related signaling in HPO-DAEE-elicited cell-death events. Overall, we concluded that HPO-DAEE induces A549-cell apoptosis through the ROS-ERK-p38 pathway and, at least in part, the CHOP pathway.


