Trimethyl phosphateCAS# 512-56-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 512-56-1 | SDF | Download SDF |
| PubChem ID | 10541 | Appearance | Powder |
| Formula | C3H9O4P | M.Wt | 140.07 |
| Type of Compound | Other Compounds | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | trimethyl phosphate | ||
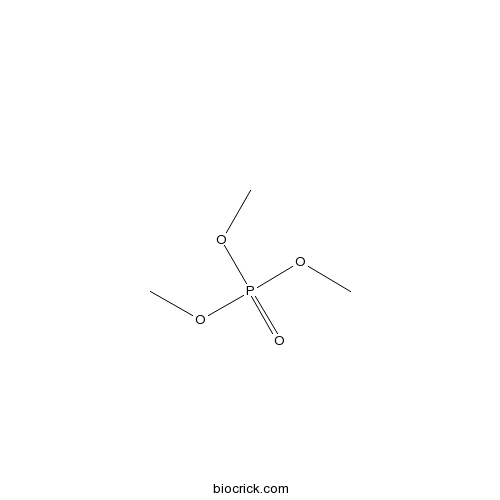
| SMILES | COP(=O)(OC)OC | ||
| Standard InChIKey | WVLBCYQITXONBZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C3H9O4P/c1-5-8(4,6-2)7-3/h1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Trimethyl phosphate Dilution Calculator

Trimethyl phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.1393 mL | 35.6964 mL | 71.3929 mL | 142.7857 mL | 178.4822 mL |
| 5 mM | 1.4279 mL | 7.1393 mL | 14.2786 mL | 28.5571 mL | 35.6964 mL |
| 10 mM | 0.7139 mL | 3.5696 mL | 7.1393 mL | 14.2786 mL | 17.8482 mL |
| 50 mM | 0.1428 mL | 0.7139 mL | 1.4279 mL | 2.8557 mL | 3.5696 mL |
| 100 mM | 0.0714 mL | 0.357 mL | 0.7139 mL | 1.4279 mL | 1.7848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Phenethylbenzamide
Catalog No.:BCN9058
CAS No.:3278-14-6
- Warfarin sodium
Catalog No.:BCN9057
CAS No.:129-06-6
- Flavokawain A
Catalog No.:BCN9056
CAS No.:37951-13-6
- 5'-Guanylic acid
Catalog No.:BCN9055
CAS No.:85-32-5
- Xanthosine
Catalog No.:BCN9054
CAS No.:146-80-5
- 5'-Cytidylic acid
Catalog No.:BCN9053
CAS No.:63-37-6
- DL-Tartaric acid
Catalog No.:BCN9052
CAS No.:133-37-9
- Mirificin-4'-O-glucoside
Catalog No.:BCN9051
CAS No.:168035-01-6
- Bisisorhapontigenin D
Catalog No.:BCN9050
CAS No.:
- Hydroquinone
Catalog No.:BCN9049
CAS No.:123-31-9
- 1αH,5αH-guaia-6-ene-4β,10β-diol
Catalog No.:BCN9048
CAS No.:2013537-81-8
- Deslanoside
Catalog No.:BCN9047
CAS No.:17598-65-1
- Glucodigifucoside
Catalog No.:BCN9060
CAS No.:2446-63-1
- (±)-Naringenin
Catalog No.:BCN9061
CAS No.:67604-48-2
- α-L-Rhamnopyranose
Catalog No.:BCN9062
CAS No.:6014-42-2
- D-Ribose
Catalog No.:BCN9063
CAS No.:50-69-1
- Alizarin 1-methyl ether
Catalog No.:BCN9064
CAS No.: 6170-06-5
- Eugenol acetate
Catalog No.:BCN9065
CAS No.:93-28-7
- Citral
Catalog No.:BCN9066
CAS No.:5392-40-5
- 10-hydroxydec-2-enoic acid
Catalog No.:BCN9067
CAS No.:14113-05-4
- Citronellal
Catalog No.:BCN9068
CAS No.:106-23-0
- Benzyl alcohol
Catalog No.:BCN9069
CAS No.:100-51-6
- (-)-Menthone
Catalog No.:BCN9070
CAS No.:14073-97-3
- (+)-Fenchone
Catalog No.:BCN9071
CAS No.:4695-62-9
Fast sample preparation for organo(fluoro)phosphate quantification approaches in lithium ion battery electrolytes by means of gas chromatographic techniques.[Pubmed:32540083]
J Chromatogr A. 2020 Aug 2;1624:461258.
Lithium ion batteries are essential power sources in portable electronics, electric vehicles and as energy storage devices for renewable energies. During harsh battery cell operation as well as at elevated temperatures, the electrolyte decomposes and inter alia organo(fluoro)phosphates are formed due to hydrolysis of the conducting salt lithium hexafluorophosphate (LiPF6). Since these phosphorus-containing decomposition products possess a potential toxicity based on structural similarities compared to chemical warfare agents, quantification is of high interest regarding safety estimates. In this study, two comprehensive approaches for the precipitation of highly concentrated PF6( ) were investigated, allowing the separation from target analytes (organo(fluoro)phosphates) and improving mass spectrometry-based quantification techniques. Trimethyl phosphate was used as a polar, non-acidic organophosphate reference substance for method development via liquid chromatography-mass spectrometry. Six solvents were examined regarding precipitation reaction and selectivity. Thermally degraded electrolytes were analyzed after precipitation by means of gas chromatography-flame ionization detector, demonstrating the applicability of the developed sample preparations. The optimized method was applied successfully without influencing any volatile and non-acidic decomposition products. Using optimized conditions, a precipitation rate of 98% PF6( ) was achieved. Consequently, a fast and easy sample preparation for gas chromatographic investigations on lithium ion battery electrolytes was implemented, applicable for routine analysis.
Inhibitory effects of organophosphate esters on carboxylesterase activity of rat liver microsomes.[Pubmed:32511959]
Chem Biol Interact. 2020 Jun 5;327:109148.
We investigated the inhibitory effects of 13 organophosphate esters (OPEs) and hydrolytic metabolites on the carboxylesterase activity of rat liver microsomes in vitro in order to examine whether there might be a potential impact on human health, and to elucidate the structure activity relationship. Among the test compounds, 2-ethylhexyl diphenyl phosphate (EDPhP) was the most potent inhibitor of carboxylesterase activity, as measured in terms of 4-nitrophenol acetate hydrolase activity, followed by tri-m-cresyl phosphate (TmCP), cresyl diphenyl phosphate (CDPhP) and triphenyl phosphate (TPhP). The IC50 values were as follows: EDPhP (IC50: 0.03 muM) > TmCP (0.4 muM) > CDPhP (0.8 muM) > TPhP (14 muM) > tris(1,3-dichloro-2-propyl) phosphate (17 muM) > tris(2-ethylhexyl) phosphate (77 muM) > tri-n-propyl phosphate (84 muM) > tris(2-chloroethyl) phosphate (104 muM) > tris(2-butoxyethyl) phosphate (124 muM) > tri-n-butyl phosphate (230 muM). The IC50 value of EDPhP was three orders of magnitude lower than that of bis(4-nitrophenyl) phosphate, which is widely used as an inhibitor of carboxylesterase. Trimethyl phosphate, triethyl phosphate and tris(2-chloroisopropyl) phosphate slightly inhibited the carboxylesterase activity; their IC50 values were above 300 muM. Lineweaver-Burk plots indicated that the inhibition by several OPEs was non-competitive. Diphenyl and monophenyl phosphates, which are metabolites of TPhP, showed weaker inhibitory effects than that of TPhP.
Binding of Organophosphorus Nerve Agents and Their Simulants to Metal Salts.[Pubmed:32506901]
ACS Appl Mater Interfaces. 2020 Jul 8;12(27):30941-30953.
Nerve agents (NAs) pose a great threat to society because they are easy to produce and are deadly in nature, which makes developing methods to detect, adsorb, and destroy them crucial. To enable the development of these methods, we report the use of first principles electronic structure calculations to understand the binding properties of NAs and NA simulants on metal salt surfaces. We report calculated Gibbs free binding energies (GBE) for four NAs (tabun (GA), sarin (GB), soman (GD), and venomous X (VX)) and five NA simulants (dimethyl methylphosphonate (DMMP), dimethyl chlorophosphate (DMCP), Trimethyl phosphate (TMP), methyl dichlorophosphate (MDCP), and di-isopropyl methylphosphonate (DIMP)) on metal perchlorate and metal nitrate salts using density functional theory. Our results indicate a general trend in the binding strength of NAs and NA simulants to metal salt surfaces: MDCP < DMCP < GA < GD approximately GB < TMP < VX approximately DMMP < DIMP. Based on their binding properties on salt surfaces, we identify the most effective simulant for each of the studied NAs as follows: DMCP for GA, TMP for GB and GD, and DMMP for VX. To illustrate the utility of the binding energies calculated in our study, we address the design of NA sensors based on the competitive binding of NAs and liquid crystalline compounds on metal salts. We compare our results with previous experimental findings and provide a list of promising combinations of liquid crystal and metal salt systems to selectively and sensitively detect NAs. Our study highlights the great value of computational chemistry for designing selective and sensitive NA sensors while minimizing the number of very dangerous experiments involving NAs.
Preparation and application of graphene oxide-based surface molecularly imprinted polymer for monolithic fiber array solid phase microextraction of organophosphate flame retardants in environmental water.[Pubmed:32505289]
J Chromatogr A. 2020 Jul 19;1623:461200.
Selectivity and high throughput are important for determination of trace level of various organophosphate flame retardants (OPFRs) in environmental matrices. In this work, three selective monolithic fibers for solid phase microextraction (SPME) were prepared and evaluated. They are graphene oxide (GO)-based surface Trimethyl phosphate (TMP) imprinted polymeric fiber (GO/TMP-IPF), GO-based surface tri (2-chloroethyl) phosphate (TCEP) imprinted polymeric fiber (GO/TCEP-IPF) and GO-based surface triphenyl phosphate (TPhP) imprinted polymeric fiber (GO/TPhP-IPF). The imprinting factors of GO/TMP-IPF for TMP, GO/TECP-IPF for TCEP and GO/TPhP-IPF for TPhP were tested as high as 4.3, 4.5, 10.3, respectively. The three fibers were bound to a stainless steel wire to assemble a GO-based surface molecularly imprinted polymeric fiber array (GO/MIP-FA). GO/MIP-FA-SPME device was coupled to gas chromatography-flame photometric detector and carried out simultaneous determination of TMP, TCEP and TPhP in environmental water. Under the optimal conditions, ultralow limits of quantification (1.7 ng L(-1)-5.0 ng L(-1)); linearity (>0.99); intra- and inter-day precision expressed as relative standard deviations for an array in the range of 4.9-8.6% and 5.8-8.2%, respectively, and array-to-array reproducibility in the range of 7.2-9.1% were obtained. The GO/MIP-FA-SPME technique was successfully applied for the determination of OPFRs in various environmental water samples, and the relative recoveries were found to be in the range from 72.4 to 112.0%.
Atomic Layer Deposition of Nitrogen-Doped Al Phosphate Coatings for Li-Ion Battery Applications.[Pubmed:32392406]
ACS Appl Mater Interfaces. 2020 Jun 10;12(23):25949-25960.
In situ nitrogen doping of aluminum phosphate has been investigated in two different plasma-enhanced atomic layer deposition (PE-ALD) processes. The first method consisted of the combination of Trimethyl phosphate plasma (TMP*) with a nitrogen plasma and trimethyl aluminum (TMA), that is, TMP*-N2*-TMA. The second method replaces TMP* with a diethylphosphoramidate plasma (i.e., DEPA*-N2*-TMA) of which the amine group could further aid nitrogen doping and/or eliminate the need for a nitrogen plasma step. At a substrate temperature of 320 degrees C, the TMP*-based process showed saturated growth (0.8 nm/cycle) of a nitrogen-doped (approximately 8 atom %) Al phosphate, while the process using DEPA* showed a similar amount of nitrogen but a significantly higher growth rate (1.4 nm/cycle). In the latter case, nitrogen doping could also be achieved without the nitrogen plasma, but this leads to a high level of carbon contamination. Both films were amorphous as-deposited, while X-ray diffraction peaks related to AlPO4 appeared after annealing in a He atmosphere. For high coating thickness (>2 nm), a significant increase in the Li-ion transmittance was found after nitrogen doping, although the coating has to be electrochemically activated. At lower thickness scales, such activation was not needed and nitrogen doping was found to double the effective transversal electronic conductivity. For the effective transversal ionic conductivity, no conclusive difference was found. When a lithium nickel manganese cobalt oxide (NMC) powder is coated with one ALD cycle of N-doped Al phosphate, the rate capability and the energy efficiency of the electrode improves.
Solvent-Free Method Prepared a Sandwich-like Nanofibrous Membrane-Reinforced Polymer Electrolyte for High-Performance All-Solid-State Lithium Batteries.[Pubmed:32302102]
ACS Appl Mater Interfaces. 2020 May 13;12(19):21586-21595.
Solid polymer electrolytes (SPEs) with the advantages of high safety, low volatility, and the ability to suppress Li dendrites are highly desirable to be used in next generation high-safety and high-energy lithium-ion batteries. The exploration of SPEs with superior comprehensive properties has received extensive attention for high-performance all-solid-state batteries (ASSBs). Herein, a sandwich-like nanofibrous membrane-reinforced poly-caprolaclone diol and Trimethyl phosphate (TMP) composite polymer electrolyte (CPE) has been designed by a facile "solvent-free" solution-casting method. Specifically, the flame-retardant TMP is employed as a plasticizer, which can improve the ionic conductivity effectively. The as-prepared solid electrolyte exhibits superior comprehensive performance in terms of high ionic conductivity, wide electrochemical window, good compatibility with lithium metal, and superior thermal stability. Furthermore, the assembled Li//LiFePO4 ASSBs with this solid CPE show outstanding cycling stability and high average discharge capacity at room temperature (30 degrees C). Undoubtedly, our study provides a new facile method and a qualified solid electrolyte material for next generation high-performance ASSBs.
In vitro oxidative stress, mitochondrial impairment and G1 phase cell cycle arrest induced by alkyl-phosphorus-containing flame retardants.[Pubmed:32006839]
Chemosphere. 2020 Jun;248:126026.
Phosphorus-containing flame retardants (PFRs) have been frequently detected in various environmental samples at relatively high concentrations and are considered emerging environmental pollutants. However, their biological effects and the underlying mechanism remain unclear, especially alkyl-PFRs. In this study, a battery of in vitro bioassays was conducted to analyze the cytotoxicity, oxidative stress, mitochondrial impairment, DNA damage and the involved molecular mechanisms of several selected alkyl-PFRs. Results showed that alkyl-PFRs induced structural related toxicity, where alkyl-PFRs with higher logKow values induced higher cytotoxicity. Long-chain alkyl-PFRs caused mitochondrial and DNA damage, resulting from intracellular reactive oxygen species (ROS) and mitochondrial superoxide overproduction; while short-chain alkyl-PFRs displayed adverse outcomes by significantly impairing mitochondria without obvious ROS generation. In addition, alkyl-PFRs caused DNA damage-induced cell cycle arrest, as determined by flow cytometry, and transcriptionally upregulated key transcription factors in p53/p21-mediated cell cycle pathways. Moreover, compared to the control condition, triisobutyl phosphate and Trimethyl phosphate exposure increased the sub-G1 apoptotic peak and upregulated the p53/bax apoptosis pathway, indicating potential cell apoptosis at the cellular and molecular levels. These results provide insight into PFR toxicity and the involved mode of action and indicate the mitochondria is an important target for some alkyl-PFRs.
A Potassium Ion-Exchanged Glass Optical Waveguide Sensor Locally Coated with a Crystal Violet-SiO2 Gel Film for Real-Time Detection of Organophosphorus Pesticides Simulant.[Pubmed:31569346]
Sensors (Basel). 2019 Sep 28;19(19). pii: s19194219.
An optical waveguide (OWG) sensor was developed for real-time detection of diethyl chlorophosphate (DCP) vapor, which is a typical simulant for organophosphorus pesticides and chemical weapon agents. Silica gel, crystal violet (CV), and potassium ion-exchange (PIE) OWG were used to fabricate the sensor's device. In the real-time detection of the DCP vapor, the volume fraction of DCP vapor was recorded to be as low as 1.68 x 10(-9). Moreover, the detection mechanism of CV-SiO2 gel film coated the PIE OWG sensor for DCP, which was evaluated by absorption spectra. These results demonstrated that the change of output light intensity of the OWG sensor significantly increased with the augment of the DCP concentration. Repeatability as well as selectivity of the sensors were tested using 0.042 x 10(-6) and 26.32 x 10(-6) volume fraction of the DCP vapor. No clear interference with the DCP detection was observed in the presence of other common solvents (e.g., acetone, methanol, dichloromethane, dimethylsulfoxide, and tetrahydrofuran), benzene series (e.g., benzene, toluene, chlorobenzene, and aniline), phosphorus-containing reagents (e.g., dimethyl methylphosphonate and Trimethyl phosphate), acid, and basic gas (e.g., acetic acid and 25% ammonium hydroxide), which demonstrates that the OWG sensor could provide real-time, fast, and accurate measurement results for the detection of DCP.
Data for quantum-chemical modeling of the mechanisms of ring-opening polymerization of methyl ethylene phosphate.[Pubmed:31528673]
Data Brief. 2019 Aug 29;26:104431.
The data presented in this paper are related to the research article entitled "Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate" (Nifant'ev et al., 2019). In this data article, we present 3D molecular information of 76 structures for TBD-catalyzed transformations of methyl ethylene phosphate (MeOEP) and Trimethyl phosphate (TMP). We also present 3D molecular information for 24 complexes that model the reaction profile of transesterification of poly(MeOEP) and TMP catalyzed by 2,6-di-tert-butyl-4-methylphenoxy magnezium species, complementing the article "Mechanistic insights of BHT-Mg-catalyzed ethylene phosphate's coordination ring-opening polymerization: DFT modeling and experimental data" (Nifant'ev et al., 2018). The data contains stationary points and transition states (TS) along the first propagation step of MeOEP ring-opening polymerization (ROP) for alternative amide and donor-acceptor mechanisms, initiated by EtOH in the presence of TBD; stationary points and TS for MeOH and HOCH2CH2OP(O)(OMe)2 initiated ROP of MeOEP; and stationary points and TS for transesterification of poly(MeOEP) and TMP. In addition, the data contains stationary points and transition states for the ROP of MeOEP and transesterification of poly(MeOEP) and TMP catalyzed by 2,6-di-tert-butylphenoxy magnesium complex. The data are provided in a PDB format that can be used for further studies.
Optimized Nonflammable Concentrated Electrolytes by Introducing a Low-Dielectric Diluent.[Pubmed:31498585]
ACS Appl Mater Interfaces. 2019 Oct 2;11(39):35770-35776.
Concentrated electrolytes of LiN(SO2F)2 (LiFSA) and organic phosphates (e.g., Trimethyl phosphate, TMP) are receiving intense attention for safe and long-lasting lithium-ion batteries, because of their nonflammable character and unusual passivation ability toward negative electrodes. However, their high viscosity and low ionic conductivity have hampered their practical application. In this work, a low-dielectric diluent, 1,1,2,2-tetrafluoroethyl 2,2,3,3,-tetrafluoropropyl ether (HFE), is introduced into concentrated LiFSA/TMP electrolytes. Upon dilution, the viscosity drastically decreases to 11.0 mPa s and the ionic conductivity slightly increases to 0.87 mS cm(-1). More importantly, both of the nonflammable character and the unusual passivation ability are retained even after dilution. A spectroscopic analysis shows that the diluted LiFSA/TMP:HFE has a local coordination state similar to that in the concentrated LiFSA/TMP, which leads to the formation of a FSA anion-derived inorganic surface film. This work suggests the importance of the peculiar local coordination state in designing safe battery electrolytes with better passivation ability.
Efficient and Selective Adsorption of Gold Ions from Wastewater with Polyaniline Modified by Trimethyl Phosphate: Adsorption Mechanism and Application.[Pubmed:30970676]
Polymers (Basel). 2019 Apr 9;11(4). pii: polym11040652.
The selective recovery of gold from wastewater is necessary because it is widely used in various fields. In this study, a new polymeric adsorbent (TP-AFC) was prepared by modifying polyaniline with Trimethyl phosphate for the selective recovery of gold from wastewater. Bath experiments were carried out to explore the adsorption capacity and mechanism. The optimum pH of adsorption is 4. The adsorption equilibrium is reached at 840 min. The maximum adsorption capacity is 881 mg/g and the adsorption was a spontaneous endothermic process. The adsorption process fitted well with pseudo second-order kinetic and the Langmuir-models. The single-layer chemisorption governed the adsorption process. In addition, the application in wastewater indicated that the interfering ions had no effect on the adsorption of gold ions. TP-AFC has good selectivity. The interaction mechanism was mainly ion exchange and complexation. In general, TP-AFC was successfully prepared and has an excellent future in practical application.
Synergetic Effect of Ethyl Methyl Carbonate and Trimethyl Phosphate on BF4(-) Intercalation into a Graphite Electrode.[Pubmed:30811939]
Langmuir. 2019 Mar 19;35(11):3972-3979.
LiBF4-ethyl methyl carbonate (EMC)-based solutions have not been successfully employed in dual-ion batteries (DIBs) mainly on account of few solvated BF4(-) intercalations into graphite positive electrodes. In this article, Trimethyl phosphate (TMP) is introduced into 1 M LiBF4-EMC solution as the electrolyte solution for DIBs, thus revealing a synergetic effect in which the discharge capacity for the anion storage using 1 M LiBF4-EMC/TMP (8:2 by vol) (ca. 26.7 mA h g(-1)) is far superior to that for the batteries using 1 M LiBF4-EMC (ca. 2.7 mA h g(-1)) or 1 M LiBF4-TMP (ca. 1.1 mA h g(-1)). In this case, the effects of TMP on LiBF4 in 1 M LiBF4-EMC/TMP electrolyte solutions are explored by conventional electrochemical tests, ex situ X-ray diffraction, in situ Raman spectroscopy, and nuclear magnetic resonance.


