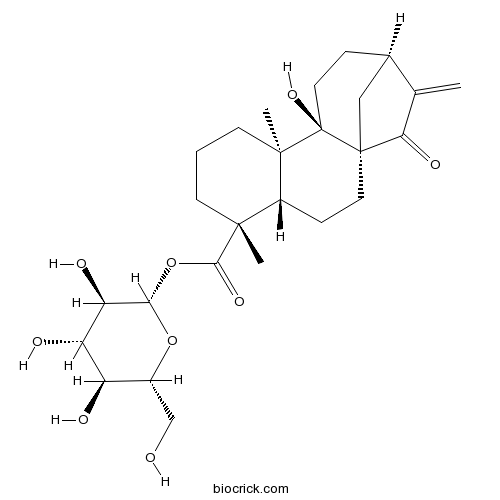
ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl esterCAS# 81263-96-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81263-96-9 | SDF | Download SDF |
| PubChem ID | 102004618 | Appearance | Powder |
| Formula | C26H38O9 | M.Wt | 494.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1R,4S,5R,9R,10R,13R)-10-hydroxy-5,9-dimethyl-14-methylidene-15-oxotetracyclo[11.2.1.01,10.04,9]hexadecane-5-carboxylate | ||
| SMILES | CC1(CCCC2(C1CCC34C2(CCC(C3)C(=C)C4=O)O)C)C(=O)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | PKAGWWDWHSCPAS-TULSSYLPSA-N | ||
| Standard InChI | InChI=1S/C26H38O9/c1-13-14-5-10-26(33)24(3)8-4-7-23(2,16(24)6-9-25(26,11-14)20(13)31)22(32)35-21-19(30)18(29)17(28)15(12-27)34-21/h14-19,21,27-30,33H,1,4-12H2,2-3H3/t14-,15-,16-,17-,18+,19-,21+,23-,24-,25+,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester Dilution Calculator

ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0218 mL | 10.1092 mL | 20.2184 mL | 40.4367 mL | 50.5459 mL |
| 5 mM | 0.4044 mL | 2.0218 mL | 4.0437 mL | 8.0873 mL | 10.1092 mL |
| 10 mM | 0.2022 mL | 1.0109 mL | 2.0218 mL | 4.0437 mL | 5.0546 mL |
| 50 mM | 0.0404 mL | 0.2022 mL | 0.4044 mL | 0.8087 mL | 1.0109 mL |
| 100 mM | 0.0202 mL | 0.1011 mL | 0.2022 mL | 0.4044 mL | 0.5055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 15-Deoxoeucosterol
Catalog No.:BCN4348
CAS No.:81241-53-4
- L-741,626
Catalog No.:BCC6886
CAS No.:81226-60-0
- Panaxynol
Catalog No.:BCN3833
CAS No.:81203-57-8
- Boc-D-Tyr(2-Br-Z)-OH
Catalog No.:BCC3464
CAS No.:81189-61-9
- Apatinib
Catalog No.:BCC5099
CAS No.:811803-05-1
- Imiloxan hydrochloride
Catalog No.:BCC6875
CAS No.:81167-22-8
- RR-src
Catalog No.:BCC6956
CAS No.:81156-93-6
- Pravastatin sodium
Catalog No.:BCC2321
CAS No.:81131-70-6
- Cilastatin sodium
Catalog No.:BCC7457
CAS No.:81129-83-1
- (Z)-Lachnophyllum lactone
Catalog No.:BCN4746
CAS No.:81122-95-4
- N-Nonyldeoxynojirimycin
Catalog No.:BCC7752
CAS No.:81117-35-3
- Racecadotril
Catalog No.:BCC4614
CAS No.:81110-73-8
- ent-6,11-Dihydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN1344
CAS No.:81263-97-0
- ent-6,9-Dihydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN1343
CAS No.:81263-98-1
- ent-6,9-Dihydroxy-15-oxo-16-kauren-19-oic acid
Catalog No.:BCN1342
CAS No.:81264-00-8
- D-AP7
Catalog No.:BCC6559
CAS No.:81338-23-0
- Amisulpride hydrochloride
Catalog No.:BCC4252
CAS No.:81342-13-4
- Schisandrin C epoxide
Catalog No.:BCN3744
CAS No.:81345-36-0
- Cyclo(Tyr-Hpro)
Catalog No.:BCN2424
CAS No.:813461-21-1
- Momordicoside F1
Catalog No.:BCN3273
CAS No.:81348-81-4
- Momordicoside L
Catalog No.:BCN3274
CAS No.:81348-83-6
- Momordicoside K
Catalog No.:BCN3272
CAS No.:81348-84-7
- Momordicoside G
Catalog No.:BCN4349
CAS No.:81371-54-2
- Seglitide
Catalog No.:BCC7191
CAS No.:81377-02-8
[Toxicological studies on sophorolipid derivatives. (II). Subacute toxicity study of polyoxypropylene (12) [2'-0-beta-D-glucopyranosyl-beta-D-glucopyranosyl) oxy-] fatty acid ester-].[Pubmed:3795299]
J Toxicol Sci. 1986 Aug;11(3):213-24.
Five groups of 12 male and 12 female rats each were fed diets containing 0, 0.06, 0.25, 1.00 and 4.00% PSL for a period of one month. Food consumption of PSL-fed groups did not differ from that of control. Urinalysis and autopsy findings were within normal in every group of rats treated. With 4.00% in the diet, body weight gain was significantly retarded and water consumption was increased, and soft stool occurred. In the hematological examination, decrease of red blood cells and increase of white blood cells were observed at the levels of 1.00 and 4.00% PSL. Changes of white blood cell differentials were also seen at the same levels. Serum Na+ concentration was slightly decreased at the 0.25, 1.00, 4.00% levels and serum glucose was also decreased at the 1.00, 4.00% levels, but the values were within the normal limits. Significant increase of relative liver weight, without histopathological changes, was observed at the 4.00% level. Histopathological examination revealed slight erosion, necrosis or intestinitis in small intestine, at the levels of 0.25, 1.00, 4.00% PSL. It was considered that these findings were attributed to the irritation potential of PSL or its metabolite. These results indicated that the non-effect level was 0.06% (53 mg/kg/day) and the level causing no toxicological effect was 0.25% (208 mg/kg/day), but no deleterious effects was observed in the levels greater than 0.25%.
Changes in abscisic acid and its beta-D-glucopyranosyl ester levels during tomato (Lycopersicon esculentum Mill.) seed development.[Pubmed:24221848]
Plant Cell Rep. 1991 Nov;10(9):444-7.
The role of abscisic acid (ABA) in tomato (Lycopersicon esculentum Mill.) zygotic embryogenesis was analysed. ABA and ABA ss-D-glucopyranosyl ester (ABA-GE) changes were determined in seeds and fruit tissues - placenta and mesocarp - during seed development, which was defined with eight embryo stages: from globular (stage 1) to mature embryo (stage 8). In whole seeds, ABA changes paralleled fresh and dry weight pattern curves and could be characterized by a high increase during embryo growth followed by a decrease as the seed matured and dehydrated. Moreover this dehydration phase led, at stage 8, to a new ABA distribution within the seed, preferentially into integument and embryo. Fruit tissue analyses provided new information about the ABA origin in seeds. ABA-GE levels were also measured and the results suggested different ABA metabolism in seed and fruit tissues.
[Toxicological studies on sophorolipid derivatives. (I). Acute toxicity, eye irritation, primary skin irritation, skin sensitization, phototoxicity, photosensitization, mutagenicity of polyoxypropylene (12) [(2'-0-beta-D-glucopyranosyl-beta-D-glucopyranosyl)oxy-] fatty acid ester-].[Pubmed:3795298]
J Toxicol Sci. 1986 Aug;11(3):197-211.
Acute toxicity, eye irritation, primary skin irritation, skin sensitization, phototoxicity, photosensitization and mutagenicity of sophorolipid derivatives were studied in rats, mice, rabbits, guinea pigs and Salmonella typhimurium strains. The acute oral toxicity of sophorolipid (SL) which Torulopsis bombicola produces, and its derivatives (PSL, Ethyl-SL and Oleyl-SL) were shown to be very low. The LD50 values of PSL ranged from 10 g/kg to 16 g/kg on oral administration in rats and mice, and from 5.8 g/kg to 6.6 g/kg on subcutaneous administration in mice. The oral LD50 values of Ethyl-SL and Oleyl-SL were estimated to be greater than 15 g/kg and that of SL was 12.5 g/kg. In eye irritation study, PSL failed to produce any reactions at 50% concentration even when the rabbit eye was not subsequently washed. SL, Ethyl-SL, Oleyl-SL and Tween 20 were "no irritant" or "slight irritant" to the rabbit eye at 20% concentration. PSL showed no irritancy to both the intact and abraded guinea pig skin at 50% concentration. And in other examinations, it was also indicated that PSL had no potentials of skin sensitization, phototoxicity and photosensitization in guinea pigs and had no potentials of mutagenicity in Salmonella typhimurium TA98 and TA100.
Sesquiterpene Lactone Composition and Cellular Nrf2 Induction of Taraxacum officinale Leaves and Roots and Taraxinic Acid beta-d-Glucopyranosyl Ester.[Pubmed:28026992]
J Med Food. 2017 Jan;20(1):71-78.
Taraxacum officinale, the common dandelion, is a plant of the Asteraceae family, which is used as a food and medical herb. Various secondary plant metabolites such as sesquiterpene lactones, triterpenoids, flavonoids, phenolic acids, coumarins, and steroids have been described to be present in T. officinale. Dandelion may exhibit various health benefits, including antioxidant, anti-inflammatory, and anticarcinogenic properties. We analyzed the leaves and roots of the common dandelion (T. officinale) using high-performance liquid chromatography/mass spectrometry to determine its sesquiterpene lactone composition. The main compound of the leaf extract taraxinic acid beta-d-glucopyranosyl ester (1), a sesquiterpene lactone, was isolated and the structure elucidation was conducted by nuclear magnetic resonance spectrometry. The leaf extract and its main compound 1 activated the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) in human hepatocytes more significantly than the root extract. Furthermore, the leaf extract induced the Nrf2 target gene heme oxygenase 1. Overall, present data suggest that compound 1 may be one of the active principles of T. officinale.


