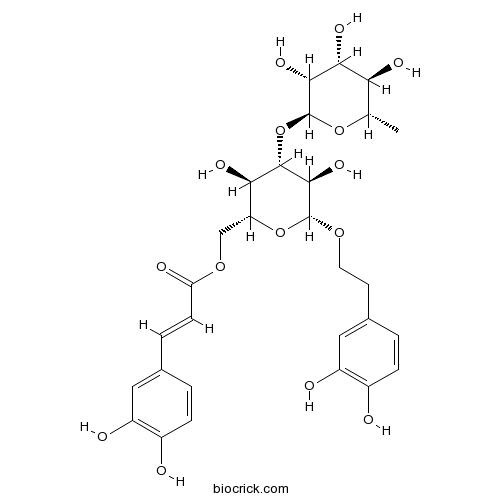
InChI=1S/C29H36O15/c1-13-22(35)24(37)25(38)29(42-13)44-27-23(36)20(12-41-21(34)7-4-14-2-5-16(30)18(32)10-14)43-28(26(27)39)40-9-8-15-3-6-17(31)19(33)11-15/h2-7,10-11,13,20,22-33,35-39H,8-9,12H2,1H3/b7-4+/t13-,20+,22-,23+,24+,25+,26+,27-,28+,29-/m0/s1
Isoacteoside, isolated from Clerodendron trichotomum (Verbenaceae), has antioxidant properties, can scavenge intracellular reactive oxygen species (ROS) and 1,1-diphenyl-
2-picrylhydrazyl (DPPH) radical, prevent lipid peroxidation, and reduce the apoptotic cells formation induced by H2O2.[1]
Isoacteoside has inhibitory activities against protein glycation in vitro may apply to cell models at higher glucose concentrations or to diabetic animal models.[2]
Isoacteoside has anti-inflammatory activity, can significantly suppress the production and mRNA expression of proinflammatory cytokines including IL-1β, IL-6, IL-8 and TNF-α in PMACI-stimulated HMC-1 cells without cytotoxicity. [3]
Isoacteoside and echinacoside stimulate the increase of α7 and α3 proteins in the cultured cells, attenuate the decreased expression of α3 and α7 nAChR subunit proteins and cell viability on SH-SY5Y cells induced by Aβ, they may play neuroprotective role by stimulating nAChR expression, which might be important in a therapeutic strategy to AD.[4]
English website: Isoacteoside
Japanese website: Isoacteoside
Chinese website: Isoacteoside
[1] Chae S, Kim J S, Kang K A, et al. J Toxicol Env Heal A, 2005, 68(5):389-400.
[2] Liu Y H, Lu Y L, Han C H, et al. Bot Stud, 2013, 54(1):1-9.
[3] Nam S Y, Kim H Y, Yoou M S, et al. Immunopharm Immunot, 2015, 37(3):1-7.
[4] Xiao-Lan Q I, Xiao H T, Xiao Y, et al. Lishizhen Medicine & Materia Medica Research, 2011, 22(7):1561-3.
[5] M X Li, Wei L L, Tao R, et al. Chinese Pharmacy, 2014 (11):1027-9.