Campsis grandiflora
Campsis grandiflora
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Campsis grandiflora
- Cat.No. Product Name CAS Number COA
-
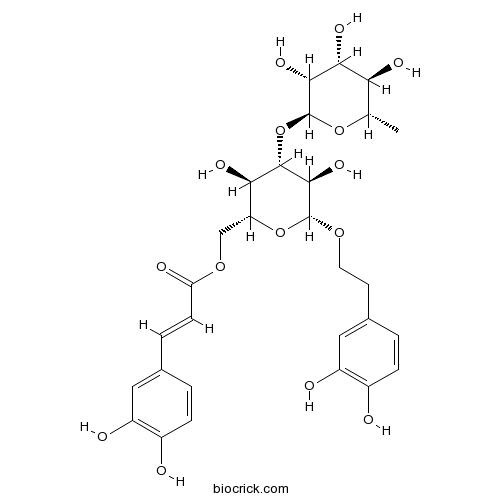
BCN4137
Isoacteoside61303-13-7
Instructions
Antidepressant-like and anti-oxidative efficacy of Campsis grandiflora flower.[Pubmed: 26408267]
Our study aimed to investigate the antidepressant-like effect of ethyl acetate extract of the flowers of Campsis grandiflora (EFCG) in a mice model of chronic unpredictable mild stress (CUMS).
Novel iridoids from the flowers of Campsis grandiflora.[Pubmed: 22370787]
A non-glycosidic iridoid, campsinol (1), and two iridoid glucosides, 7-O-(Z)-p-coumaroylcachineside V (2) and 7-O-(E)-p-coumaroylcachineside I (3), were isolated from the fresh flowers of Campsis grandiflora along with five known iridoid glycosides, ixoroside (4), campsiside (5), cachineside I (6), 5-hydroxycampenoside (7), and 5-hydroxycampsiside (8), and two known phenylpropanoid glycosides, acteoside (9) and leucosceptoside A (10). The structures of these compounds were determined based on the NMR and Mass spectroscopic data and other chemical evidences.
[Simultaneous determination of acteoside, oleanolic acid and ursolic acid in flower of Campsis grandiflora by HPLC].[Pubmed: 21809582]
To develop an HPLC method for the determination of acteoside, oleanolic acid and ursolic acid in flowers of Campsis grandiflora.
Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator.[Pubmed: 17201692]
Five pentacyclic triterpenoids isolated from Campsis grandiflora were tested for insulin-mimetic and insulin-sensitizing activity. The compounds enhanced the activity of insulin on tyrosine phosphorylation of the IR (insulin receptor) beta-subunit in CHO/IR (Chinese-hamster ovary cells expressing human IR). Among the compounds tested, CG7 (ursolic acid) showed the greatest enhancement and CG11 (myrianthic acid) the least. We characterized the effect of CG7 further, and showed that it acted as an effective insulin-mimetic agent at doses above 50 mug/ml and as an insulin-sensitizer at doses as low as 1 mug/ml. Additional experiments showed that CG7 increased the number of IRs that were activated by insulin. This indicates that a major mechanism by which CG7 enhances total IR auto-phosphorylation is by promoting the tyrosine phosphorylation of additional IRs. CG7 not only potentiated insulin-mediated signalling (tyrosine phosphorylation of the IR beta-subunit, phosphorylation of Akt and glycogen synthase kinase-3beta), but also enhanced the effect of insulin on translocation of glucose transporter 4 in a classical insulin-sensitive cell line, 3T3-L1 adipocytes. The results of the present study demonstrate that a specific pentacyclic triterpenoid, CG7, exerts an insulin-sensitizing effect as an IR activator in CHO/IR cells and adipocytes. The enhancement of insulin activity by CG7 may be useful for developing a new class of specific IR activators for treatment of Type 1 and Type 2 diabetes.
Antioxidative and acute anti-inflammatory effects of Campsis grandiflora flower.[Pubmed: 16169696]
The present study was undertaken to investigate the antioxidative and anti-inflammatory activities of the extract of the flower of Campsis grandiflora (Thunb.) K. Schum. Exposure of human dermal fibroblasts to 50% EtOH extract of Campsis grandiflora flower (ECG) at 10 and 100 microg/ml showed significant protective effect against hydrogen peroxide (300 microM). ECG not only protected cell survival from H(2)O(2)-induced toxicity, but also inhibited the H(2)O(2)-induced leakage of lactate dehydrogenase (LDH) enzyme release and DNA fragmentation significantly. It was also found that ECG showed scavenging activities of radicals and reactive oxygen species with IC(50) values of 20 microg/ml against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and 52 microg/ml against superoxide radicals in the xanthine/xanthine oxidase system, respectively. Topically applied ECG dose-dependently inhibited arachidonic acid (AA)- and 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced ear edema in mice. Consistent with its antioxidative properties in vitro, the present results suggest the therapeutic potential of ECG for acute skin inflammation that may involve oxidative tissue damage.
Triterpenoids from the flower of Campsis grandiflora K. Schum. as human acyl-CoA: cholesterol acyltransferase inhibitors.[Pubmed: 15974441]
The flower of Campsis grandiflora K. Schum. was extracted with 80% aqueous MeOH, and the concentrated extract was partitioned with EtOAc, n-BuOH and H2O. From the EtOAc fraction, seven triterpenoids were isolated through the repeated silica gel, ODS column chromatographies and preparative HPLC. From the result of physico-chemical data including NMR, MS and IR, the chemical structures of the compounds were determined as 3beta-hydroxyolean-12-en-28-oic acid (oleanolic acid, 1), 3beta-hydroxyurs-12-en-28-oic acid (ursolic acid, 2), 3beta-hydroxyurs-12-en-28-al (ursolic aldehyde, 3), 2alpha,3beta-dihydroxyolean-12-en-28-oic acid (maslinic acid, 4), 2alpha,3beta-dihydroxyurs-12-en-28-oic acid (corosolic acid, 5), 3beta,23-dihydroxyurs-12-en-28-oic acid (23-hydroxyursolic acid, 6) and 2alpha,3beta,23-trihydroxyolean-12-en-28-oic acid (arjunolic acid, 7). These teriterpenoids were isolated for the first time from this plant. Also, compounds 4, 5, 6, and 7 revealed relatively high hACAT-1 inhibitory activity with the value of 46.2+/-1.1, 46.7+/-0.9, 41.5+/-1.3 and 60.8+/-1.1% at the concentration of 100 microg/mL, respectively.
Two new non-glycosidic iridoids from the leaves of Campsis grandiflora.[Pubmed: 15971135]
Two new non-glycosidic iridoids, which were named cachinol (1) and 1-O-methyl cachinol (2), were isolated from the methanol extract of the leaves of Campsis grandiflora together with a known iridoid cachineside I (3). The structures of compounds 1 and 2 were determined on the basis of spectroscopic methods including two dimensional NMR and high resolution mass spectrometry. All of the isolated compounds showed mild inhibitory activities on rat platelet aggregation. Compounds 1 and 3 (IC50 : 246 and 219 microM, respectively) showed about 2-fold higher inhibitory effects than acetylsalicylic acid (ASA, IC50: 412 microM) on collagen-induced aggregation. Compounds 1 and 2 (IC50: 43.2 and 38.4 microM, respectively) were about 2-fold more inhibitory than ASA (IC50: 75.2 microM), and about 4-fold more effective than their glycoside 3 (IC50: 189 microM) on AA-induced aggregation.
Anti-platelet pentacyclic triterpenoids from leaves of Campsis grandiflora.[Pubmed: 15180300]
Five pentacyclic triterpenoids, oleanolic acid (1), hederagenin (2), ursolic acid (3), tormentic acid (4) and myrianthic acid (5), were isolated from the methanol extract of the leaves of Campsis grandiflora, and structures of the compounds were established by the spectroscopic methods. Compounds 2, 3, 4, and 5 were isolated for the first time from the genus Campsis. All of the compounds (IC50: 45.3, 32.8, 82.6, 42.9 and 46.2 microM respectively) were as equivalently inhibitive as acetylsalicylic acid (IC50: 57.0 microM) on epinephrine induced platelet aggregation.


