ZolpidemCAS# 82626-48-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82626-48-0 | SDF | Download SDF |
| PubChem ID | 5732 | Appearance | Powder |
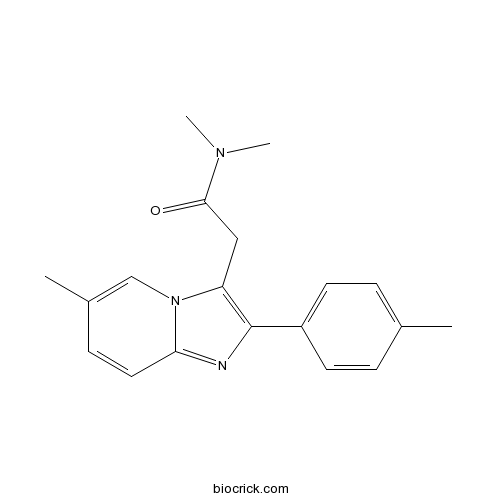
| Formula | C19H21N3O | M.Wt | 307.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N,N-dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide | ||
| SMILES | CC1=CC=C(C=C1)C2=C(N3C=C(C=CC3=N2)C)CC(=O)N(C)C | ||
| Standard InChIKey | ZAFYATHCZYHLPB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzodiazepine agonist with high selectivity for α1-subunit-containing GABAA receptors (BZ/ω1 site) and very high intrinsic activity. Ki values are 20, 400 and ≥ 5000 nM for α1-, α2-/α3-, and α5-containing GABAA receptors respectively. Hypnotic that does not induce physical dependence. |

Zolpidem Dilution Calculator

Zolpidem Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2531 mL | 16.2655 mL | 32.5309 mL | 65.0618 mL | 81.3273 mL |
| 5 mM | 0.6506 mL | 3.2531 mL | 6.5062 mL | 13.0124 mL | 16.2655 mL |
| 10 mM | 0.3253 mL | 1.6265 mL | 3.2531 mL | 6.5062 mL | 8.1327 mL |
| 50 mM | 0.0651 mL | 0.3253 mL | 0.6506 mL | 1.3012 mL | 1.6265 mL |
| 100 mM | 0.0325 mL | 0.1627 mL | 0.3253 mL | 0.6506 mL | 0.8133 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pseudolaric Acid C
Catalog No.:BCN2856
CAS No.:82601-41-0
- Mecamylamine hydrochloride
Catalog No.:BCC7501
CAS No.:826-39-1
- Triacetonamine
Catalog No.:BCN4368
CAS No.:826-36-8
- Sarsaponin
Catalog No.:BCN8293
CAS No.:82597-74-8
- Quinapril HCl
Catalog No.:BCC5011
CAS No.:82586-55-8
- Moexipril HCl
Catalog No.:BCC5015
CAS No.:82586-52-5
- Ozagrel
Catalog No.:BCC2298
CAS No.:82571-53-7
- Fmoc-Phe(4-I)-OH
Catalog No.:BCC3261
CAS No.:82565-68-2
- Myoscorpine
Catalog No.:BCN1973
CAS No.:82535-76-0
- 5-Methyl-7-methoxyisoflavone
Catalog No.:BCN8465
CAS No.:82517-12-2
- 10-Hydroxy-16-epiaffinine
Catalog No.:BCN4001
CAS No.:82513-70-0
- Deacetylpseudolaric acid A
Catalog No.:BCN4366
CAS No.:82508-37-0
- Raloxifene HCl
Catalog No.:BCC4488
CAS No.:82640-04-8
- Picrasidine A
Catalog No.:BCN4369
CAS No.:82652-20-8
- Boc-Tryptophanol
Catalog No.:BCC2700
CAS No.:82689-19-8
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- Danusertib (PHA-739358)
Catalog No.:BCC2172
CAS No.:827318-97-8
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
- Nefazodone hydrochloride
Catalog No.:BCC7479
CAS No.:82752-99-6
- H-D-HoPhe-OH
Catalog No.:BCC3239
CAS No.:82795-51-5
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
The effects of zolpidem in obstructive sleep apnea - An open-label pilot study.[Pubmed:30968498]
J Sleep Res. 2019 Apr 10:e12853.
New knowledge on hypnotics and their effects on the phenotypic causes of obstructive sleep apnea indicate that Zolpidem has therapeutic potential for certain patients. Specifically, Zolpidem increases the threshold for arousal threshold and pharyngeal dilator muscle responsiveness. However, the effects of a standard dose of Zolpidem (10 mg) on obstructive sleep apnea severity and symptoms have not been investigated. In an open-label pilot study, 12 unselected people with obstructive sleep apnea were recruited following a diagnostic in-laboratory sleep study. Participants then returned for a single-night sleep study in which 10 mg of Zolpidem was given just prior to sleep. Tolerability, next-day sleepiness and the effects of Zolpidem on polysomnography variables were assessed. Zolpidem was well tolerated and significantly improved the sleep efficiency compared with the no-drug night (77 +/- 12% versus 84 +/- 9%, p = 0.005). Individual responses on obstructive sleep apnea severity to Zolpidem in this unselected obstructive sleep apnea patient population were variable with no overall systematic difference in apnea-hypopnea index (29 +/- 18.2 events per hr versus 33 +/- 28 events per hr, p = 0.45) or other key respiratory parameters (e.g. event duration or hypoxemia). Next-day sleepiness assessed via the Karolinska Sleepiness Scale was not different between visits (4 +/- 1 versus 4 +/- 2, p = 0.85). These findings provide the first insight into the effects of a standard dose of Zolpidem in obstructive sleep apnea, and highlight its tolerability and potential to improve sleep quality. The variable effects on obstructive sleep apnea severity observed in this pilot also underscore the need for larger trials that incorporate phenotypic characterisation (e.g. arousal threshold, Pcrit and muscle responsiveness) to understand inter-individual heterogeneity and the therapeutic potential of Zolpidem for certain people with obstructive sleep apnea.
Zolpidem and Gender: Are Women Really At Risk?[Pubmed:30939589]
J Clin Psychopharmacol. 2019 Apr 1.
BACKGROUND: In 2013 the Food and Drug Administration (FDA) claimed the existence of new data showing women to be at risk for excessive daytime sedation and impaired driving proficiency following bedtime doses of Zolpidem. The putative explanation was the reduced metabolic clearance of Zolpidem and higher morning blood concentrations in women compared to men. The FDA acted to reduce the recommended dosage for women down to 50% of the dose for men. No other regulatory agency worldwide has taken similar action. METHODS: Gender effects on Zolpidem pharmacokinetics, pharmacodynamics, adverse effects, clinical efficacy, and driving performance were evaluated through a further analysis of data from a previous study, together with a literature review. RESULTS: Women had on average 35% lower apparent clearance of Zolpidem than men (236 vs 364 mL/min, P < 0.001). This difference was not explained by body weight. In some laboratory studies, women had greater functional impairment than men taking the same dose, but in all studies active drug was not distinguishable from placebo at 8 hours after oral dosage. On-the-road driving studies likewise showed no evidence of driving impairment in men or women at 8 hours after 10 mg of oral immediate-release Zolpidem. No clinical trial demonstrated a gender-related difference in clinical efficacy or adverse reactions, and there was no evidence of a particular risk to women. CONCLUSIONS: Dosage reduction in women is not supported by available scientific evidence, and may in fact lead to underdosing and the consequent hazard of inadequately treated insomnia.
Zolpidem and Zolpidem Phenyl-4-carboxylic Acid Pharmacokinetics in Oral Fluid after a Single Dose.[Pubmed:30912258]
Drug Test Anal. 2019 Mar 25.
BACKGROUND: Oral fluid Zolpidem detection in the settings of drug-facilitated crime and roadside drug testing indicates recent Zolpidem intake. Zolpidem pharmacokinetics in classical biological matrices such as blood and urine have been described; however, reports of such data based on oral fluids are limited. OBJECTIVE: The aim of this study is to describe the pharmacokinetics of Zolpidem and its major metabolite Zolpidem phenyl-4-carboxylic acid (ZPCA) in oral fluids after intake. METHODS: Ten milligrams of Zolpidem tartrate tablets were orally administered to 14 volunteers, and oral fluid samples were collected at various times up to 72 h and analyzed via LC-MS/MS with post-column reagent addition. RESULTS: Both Zolpidem and ZPCA could be detected in oral fluid after 1 hour and were rapidly eliminated, with half-lives of 2.77+/-0.71 h and 5.11+/-0.67 h, respectively. Maximum Zolpidem concentrations (36.73+/-10.89 ng/mL) occurred at 2+/-0.52 h, and maximum ZPCA concentrations (0.28+/-0.16 ng/mL) occurred at 2+/-0.37 h. Zolpidem/ZPCA ratios decreased after Zolpidem intake, an observation that might be helpful for determining the time of drug use. CONCLUSION: The results showed that the measurement of Zolpidem in oral fluid can be used for the noninvasive monitoring of Zolpidem consumption and misuse in drug-facilitated crime and roadside drug testing settings.
Comorbid Zolpidem Dependence and Over-the-Counter Compound Analgesic Abuse.[Pubmed:30905134]
Clin Psychopharmacol Neurosci. 2019 May 31;17(2):323-325.
Zolpidem is a commonly prescribed hypnotic used to treat insomnia. However, its potential for abuse and dependence has recently become controversial. Although over-the-counter (OTC) medications are widely used, their abuse potential has not received much research attention. We report a case of comorbid Zolpidem and OTC compound analgesic abuse. OTC analgesics may serve as gateway drugs, and physicians must be cautious about this issue, especially when prescribing hypnotics or benzodiazepines.
Visual Hallucinations from Zolpidem Use for the Treatment of Hospital Insomnia in a Septuagenarian.[Pubmed:30891388]
Cureus. 2019 Jan 8;11(1):e3848.
Insomnia and parasomnias are common patient complaints during hospital stay. New environment, the severity of underlying disease, level of care, medications, and infections are all known factors that contribute toward insomnia. Zolpidem is a common sleep aid used for this purpose. We report a case of Zolpidem-induced visual hallucination in a septuagenarian in the inpatient setting.
Zolpidem Use and Suicide Death in South Korea: A Population-Based Case-Control Study.[Pubmed:30883921]
Suicide Life Threat Behav. 2019 Mar 18.
OBJECTIVE: To investigate whether Zolpidem use is associated with suicide death in adults. METHOD: We conducted a case-control study using the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database. Cases were adults with a suicide record (ICD-10 codes; X-60-X84, Y87.0) between January 1, 2004 and December 31, 2013. 10 Controls were matched to each case by age, sex, index year, region, income level, and health insurance type. Zolpidem use during 2 years before suicide was quantified. Adjusted odd ratios (aORs) with 95% confidence intervals (CIs) were estimated using conditional logistic regression. RESULTS: The percentage of Zolpidem users was significantly higher in cases (451 of 1,928 [23.4%]) than in controls (832 of 18,404 [4.5%]). After controlling for potential confounders, Zolpidem use was significantly associated with suicide (aORs, 2.09; 95% CI, 1.74-2.52). Dose-response relationships were observed (for trend, p < .0001). Consistent findings were observed when analyses were restricted to suicide death (aORs, 2.08; 95% CI, 1.73-2.51) and nonmedication poisoning suicide death cases (aORs, 2.10; 95% CI, 1.74-2.53). CONCLUSIONS: We found a significant and positive association between Zolpidem use and suicide. Zolpidem should be prescribed cautiously and with due caution of increased suicide risk.
Psychiatric Disorders and Oxidative Injury: Antioxidant Effects of Zolpidem Therapy disclosed In Silico.[Pubmed:30867894]
Comput Struct Biotechnol J. 2019 Feb 7;17:311-318.
Zolpidem (N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide) is a well-known drug for the treatment of sleeping disorders. Recent literature reports on positive effects of Zolpidem therapy on improving renal damage after cisplatin and on reducing akinesia without sleep induction. This has been ascribed to the antioxidant and neuroprotective capacity of this molecule, and tentatively explained according to a generic structural similarity between Zolpidem and melatonin. In this work, we investigate in silico the antioxidant potential of Zolpidem as scavenger of five ROSs, acting via hydrogen atom transfer (HAT) mechanism; computational methodologies based on density functional theory are employed. For completeness, the analysis is extended to six metabolites. Thermodynamic and kinetic results disclose that indeed Zolpidem is an efficient radical scavenger, similarly to melatonin and Trolox, supporting the biomedical evidence that the antioxidant potential of Zolpidem therapy may have a beneficial effect against oxidative injury, which is emerging as an important etiopathogenesis in numerous severe diseases, including psychiatric disorders.
Mechanism of action of the hypnotic zolpidem in vivo.[Pubmed:11090095]
Br J Pharmacol. 2000 Dec;131(7):1251-4.
Zolpidem is a widely used hypnotic agent acting at the GABA(A) receptor benzodiazepine site. On recombinant receptors, Zolpidem displays a high affinity to alpha 1-GABA(A) receptors, an intermediate affinity to alpha(2)- and alpha(3)-GABA(A) receptors and fails to bind to alpha(5)-GABA(A) receptors. However, it is not known which receptor subtype is essential for mediating the sedative-hypnotic action in vivo. Studying alpha1(H101R) mice, which possess Zolpidem-insensitive alpha(1)-GABA(A) receptors, we show that the sedative action of Zolpidem is exclusively mediated by alpha(1)-GABA(A) receptors. Similarly, the activity of Zolpidem against pentylenetetrazole-induced tonic convulsions is also completely mediated by alpha(1)-GABA(A) receptors. These results establish that the sedative-hypnotic and anticonvulsant activities of Zolpidem are due to its action on alpha(1)-GABA(A) receptors and not on alpha(2)- or alpha(3)-GABA(A) receptors.
Lack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidem.[Pubmed:1403792]
J Pharmacol Exp Ther. 1992 Oct;263(1):298-303.
Zolpidem is a new, short-acting hypnotic of imidazopyridine structure which binds selectively to a subpopulation of receptors involved in the action of benzodiazepines [omega 1 (BZ1) sites of the gamma-aminobutyric acidA receptors]. The present study investigated whether tolerance and physical dependence develop after repeated treatment with Zolpidem as is observed with benzodiazepines. Mice were given Zolpidem or the benzodiazepine midazolam (2 x 30 mg/kg, p.o.) for 10 consecutive days. Tolerance to central depressant effects (evaluated by recording spontaneous locomotor activity) and to anticonvulsant effects (measured against pentylenetetrazole-, electroshock- and isoniazid-induced convulsions) was assessed 42 hr after the last administration. A decrease in the latency to isoniazid-induced convulsions was taken as an index of physical dependence and was evaluated 3, 6, 14, 24, 42 and 67 hr after the end of chronic drug treatment. Repeated treatment with midazolam produced tolerance to its sedative and anticonvulsant activities as indicated by shifts of the dose-response curves by a factor of 3 to 5. Fourteen hr after discontinuation of treatment, spontaneous withdrawal was observed and lasted 3 days. When flumazenil was given 3 or 6 hr after the final midazolam injection, precipitated withdrawal was observed. In contrast, after repeated treatment with Zolpidem, there was no change in its ability to produce sedative and anticonvulsant effects. Moreover, neither spontaneous nor flumazenil-induced precipitated withdrawal was observed in Zolpidem-treated mice.
High affinity [3H]zolpidem binding in the rat brain: an imidazopyridine with agonist properties at central benzodiazepine receptors.[Pubmed:2878820]
Eur J Pharmacol. 1986 Nov 4;130(3):257-63.
[3H]Zolpidem, a novel hypnotic drug possessing a chemical structure unrelated to that of benzodiazepine (BZD) was employed as a new ligand to determine its binding characteristics to membrane preparations of rat cerebral cortex and cerebellum. In both structures, the imidazopyridine [3H]Zolpidem bound with high affinity to a single population of recognition sites. The cerebellum possessed a similar number of [3H]Zolpidem and [3H]diazepam binding sites, while the cerebral cortex possessed a lower density of [3H]Zolpidem than [3H]diazepam binding sites. In contrast to [3H]diazepam binding, [3H]Zolpidem binding was not detectable in the spinal cord. In the cortex, BZDs had a similar potency to displace [3H]Zolpidem and [3H]diazepam binding while non-BZDs were more potent to inhibit [3H] Zolpidem binding than [3H]diazepam binding. The binding of [3H]Zolpidem was enhanced by GABA to the same extent as [3H]diazepam binding. The increase in [3H] Zolpidem binding caused by chloride ions was less pronounced than that in [3H]diazepam binding. It is concluded that [3H]Zolpidem possesses selectivity for BZD receptors with the pharmacological characteristics and regional distribution of the BZD1 receptor subtype. [3H]Zolpidem as a radioligand offers a useful additional tool to study the mechanism of action of hypnotics acting through BZD receptor subtypes.
Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects.[Pubmed:2871178]
J Pharmacol Exp Ther. 1986 May;237(2):649-58.
Zolpidem [N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide hemitartrate] is reported to be a rapid onset, short duration hypnotic that interacts at the benzodiazepine recognition site. The present report establishes the neuropsychopharmacological profile of Zolpidem and compares it with those of benzodiazepine hypnotics. Although in mice the effects of Zolpidem are qualitatively similar to those of midazolam, triazolam and flunitrazepam, sedation with Zolpidem occurs at doses 10 and 20 times lower than those inducing anticonvulsant and myorelaxant effects, respectively. In contrast, the benzodiazepines studied induce sedation at doses causing myorelaxation and which are 2 to 6 times superior to those antagonizing pentetrazole-induced convulsions. In the rat, Zolpidem induces sleep (as indicated behaviorally and electrocorticographically) and displays anticonflict activity in a punished drinking paradigm, as do the benzodiazepines. However, whereas benzodiazepine hypnotics induce EEG sleep patterns in curarized rats at doses similar or inferior to those active in the conflict test (in freely moving animals), the hypnotic effect of Zolpidem is seen at doses 10 times lower than those producing an anticonflict effect. Moreover, a qualitative difference between the effects of Zolpidem and benzodiazepines is observed in electrocorticographic recordings obtained in curarized rats: electrocorticographic hypersynchronization induced by Zolpidem is dominated by the energy increase within the 2 to 4 Hz band whereas the benzodiazepines increase predominantly energy levels within the 12 to 14 Hz band. Studies of the sleep-wakefulness cycle in the rat and the cat revealed that hypnotic doses of Zolpidem do not alter the pattern of physiological sleep, although elevated doses of the drug decrease paradoxical sleep and increase slow wave sleep. In rats trained to discriminate chlordiazepoxide, Zolpidem fails to generalize with the chlordiazepoxide-associated lever indicating that the compound and benzodiazepines do not share the same discriminative stimulus properties. Nevertheless, the anticonvulsant, hypnotic, myorelaxant and anticonflict effects of Zolpidem are antagonized by benzodiazepine receptor antagonist Ro 15-1788 and CGS 8216 indicating an involvement of the benzodiazepine recognition site in the action of this drug. The highly selective sedative effect of Zolpidem (as compared to myorelaxant and anticonvulsant effects) suggests that it may possess a specificity for certain subtypes of benzodiazepine receptors.


