TriacetonamineCAS# 826-36-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 826-36-8 | SDF | Download SDF |
| PubChem ID | 13220 | Appearance | Powder |
| Formula | C9H17NO | M.Wt | 155.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
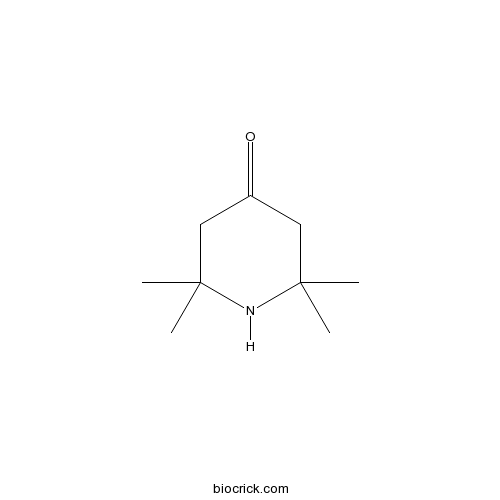
| Chemical Name | 2,2,6,6-tetramethylpiperidin-4-one | ||
| SMILES | CC1(CC(=O)CC(N1)(C)C)C | ||
| Standard InChIKey | JWUXJYZVKZKLTJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H17NO/c1-8(2)5-7(11)6-9(3,4)10-8/h10H,5-6H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triacetonamine protects the activitis of superoxide dismutase(SOD) and creatine phos-phate kinase (CPK); it also inhibits CaCl2 induced agglomeration of thromocyte. Triacetonamine can improve the constructile function of heart mus-cles of acute anemia and decrease the content of malodialdehyde (MDA) in the heart muscles. |
| Targets | SOD | MDA |
| In vitro | Triacetonamine formation in a bio-oil from fast pyrolysis of sewage sludge using acetone as the absorption solvent.[Pubmed: 20137920]Bioresour Technol. 2010 Jun;101(11):4242-5.A sewage sludge sample was pyrolyzed in a drop tube furnace at 500 degrees C and sweeping gas flow rate of 300cm(3)/min. Protective Effects of Triacetonamine on Acute Ischemia Isolated Rat Heart Muscles[Reference: WebLink]Supplement to the Journal of Sun Yatsen University, 1994 (6) :9-11.Triacetonamine(TAA)could improve the constructile function of heart mus-cles of acute anemia and decrease the content of malodialdehyde (MDA) in the heart muscles. TAA protects the activitis of superoxide dismutase(SOD) and creatine phos-phate kinase (CPK). It also inhibits CaCl2 induced agglomeration of thromocyte. |
| Structure Identification | J Comput Aided Mol Des. 1990 Dec;4(4):403-9.Theoretical models for the conformations and the protonation of triacetonamine.[Pubmed: 1965442]In this paper we propose theoretical models for the conformations of Triacetonamine and protonated Triacetonamine (Vincubine, an anticancer chemotherapeutic agent) developed by quantum and molecular mechanics techniques. |

Triacetonamine Dilution Calculator

Triacetonamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4433 mL | 32.2165 mL | 64.433 mL | 128.866 mL | 161.0825 mL |
| 5 mM | 1.2887 mL | 6.4433 mL | 12.8866 mL | 25.7732 mL | 32.2165 mL |
| 10 mM | 0.6443 mL | 3.2216 mL | 6.4433 mL | 12.8866 mL | 16.1082 mL |
| 50 mM | 0.1289 mL | 0.6443 mL | 1.2887 mL | 2.5773 mL | 3.2216 mL |
| 100 mM | 0.0644 mL | 0.3222 mL | 0.6443 mL | 1.2887 mL | 1.6108 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sarsaponin
Catalog No.:BCN8293
CAS No.:82597-74-8
- Quinapril HCl
Catalog No.:BCC5011
CAS No.:82586-55-8
- Moexipril HCl
Catalog No.:BCC5015
CAS No.:82586-52-5
- Ozagrel
Catalog No.:BCC2298
CAS No.:82571-53-7
- Fmoc-Phe(4-I)-OH
Catalog No.:BCC3261
CAS No.:82565-68-2
- Myoscorpine
Catalog No.:BCN1973
CAS No.:82535-76-0
- 5-Methyl-7-methoxyisoflavone
Catalog No.:BCN8465
CAS No.:82517-12-2
- 10-Hydroxy-16-epiaffinine
Catalog No.:BCN4001
CAS No.:82513-70-0
- Deacetylpseudolaric acid A
Catalog No.:BCN4366
CAS No.:82508-37-0
- Demethoxydeacetoxypseudolaric acid B
Catalog No.:BCN4365
CAS No.:82508-36-9
- Methyl pseudolarate B
Catalog No.:BCN4364
CAS No.:82508-34-7
- Methyl pseudolarate A
Catalog No.:BCN4363
CAS No.:82508-33-6
- Mecamylamine hydrochloride
Catalog No.:BCC7501
CAS No.:826-39-1
- Pseudolaric Acid C
Catalog No.:BCN2856
CAS No.:82601-41-0
- Zolpidem
Catalog No.:BCC9194
CAS No.:82626-48-0
- Raloxifene HCl
Catalog No.:BCC4488
CAS No.:82640-04-8
- Picrasidine A
Catalog No.:BCN4369
CAS No.:82652-20-8
- Boc-Tryptophanol
Catalog No.:BCC2700
CAS No.:82689-19-8
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- Danusertib (PHA-739358)
Catalog No.:BCC2172
CAS No.:827318-97-8
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
Theoretical models for the conformations and the protonation of triacetonamine.[Pubmed:1965442]
J Comput Aided Mol Des. 1990 Dec;4(4):403-9.
In this paper we propose theoretical models for the conformations of Triacetonamine and protonated Triacetonamine (Vincubine, an anticancer chemotherapeutic agent) developed by quantum and molecular mechanics techniques. We discuss the theoretical factors which are involved in the stabilization of the conformations calculated by the MNDO, MM2 and COPEANE methods and show the relative percent abundance of each molecular shape. Graphic representations of the conformers are depicted.
Triacetonamine formation in a bio-oil from fast pyrolysis of sewage sludge using acetone as the absorption solvent.[Pubmed:20137920]
Bioresour Technol. 2010 Jun;101(11):4242-5.
A sewage sludge sample was pyrolyzed in a drop tube furnace at 500 degrees C and sweeping gas flow rate of 300cm(3)/min. Triacetonamine (TAA) was detected with GC/MS as major component in the resulting bio-oil using acetone as the absorption solvent and proven to be a product from the reaction of NH(3) in the bio-oil with the absorption solvent acetone. TAA yield increased with storage time and reached a level about 28.4% (% sludge fed, daf) after 175h. Since the reaction of pure NH(3) with acetone does not proceed, some species in the bio-oil must catalyze the reaction of NH(3) with acetone. TAA was isolated in a high yield (27.9%, daf) and high purity (80.4%) by column chromatography with different solvents, including mixed solvents, as eluants. The study revealed the possibility of sewage sludge as potential resource of TAA.


