Withaperuvin CCAS# 81644-34-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81644-34-0 | SDF | Download SDF |
| PubChem ID | 11092126 | Appearance | Powder |
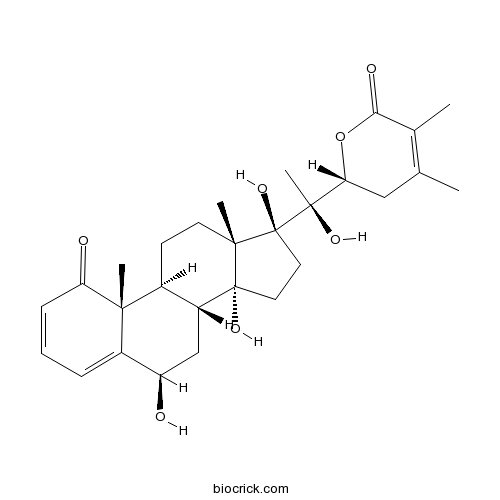
| Formula | C28H38O7 | M.Wt | 486.59 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-[(1S)-1-hydroxy-1-[(6R,8R,9S,10R,13S,14R,17S)-6,14,17-trihydroxy-10,13-dimethyl-1-oxo-6,7,8,9,11,12,15,16-octahydrocyclopenta[a]phenanthren-17-yl]ethyl]-4,5-dimethyl-2,3-dihydropyran-6-one | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)(C2(CCC3(C2(CCC4C3CC(C5=CC=CC(=O)C45C)O)C)O)O)O)C | ||
| Standard InChIKey | IBJZGHYOMSKIJB-TWLFGGHSSA-N | ||
| Standard InChI | InChI=1S/C28H38O7/c1-15-13-22(35-23(31)16(15)2)26(5,32)28(34)12-11-27(33)19-14-20(29)18-7-6-8-21(30)25(18,4)17(19)9-10-24(27,28)3/h6-8,17,19-20,22,29,32-34H,9-14H2,1-5H3/t17-,19+,20+,22+,24-,25+,26-,27+,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | TNF-α | NF-kB |

Withaperuvin C Dilution Calculator

Withaperuvin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0551 mL | 10.2756 mL | 20.5512 mL | 41.1024 mL | 51.378 mL |
| 5 mM | 0.411 mL | 2.0551 mL | 4.1102 mL | 8.2205 mL | 10.2756 mL |
| 10 mM | 0.2055 mL | 1.0276 mL | 2.0551 mL | 4.1102 mL | 5.1378 mL |
| 50 mM | 0.0411 mL | 0.2055 mL | 0.411 mL | 0.822 mL | 1.0276 mL |
| 100 mM | 0.0206 mL | 0.1028 mL | 0.2055 mL | 0.411 mL | 0.5138 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neuromedin C (porcine)
Catalog No.:BCC5832
CAS No.:81608-30-2
- 2-Pentadecenedioic acid
Catalog No.:BCN3666
CAS No.:81588-35-4
- Forsythoside B
Catalog No.:BCN1205
CAS No.:81525-13-5
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- alpha-Dihydroartemisinin
Catalog No.:BCN2627
CAS No.:81496-81-3
- 1-Hydroxypinoresinol 1-O-glucoside
Catalog No.:BCN7019
CAS No.:81495-71-8
- Taxagifine
Catalog No.:BCN6949
CAS No.:81489-69-2
- (-)-Pinoresinol
Catalog No.:BCN3254
CAS No.:81446-29-9
- 8-Hydroxypinoresinol
Catalog No.:BCN3389
CAS No.:81426-17-7
- 8-Acetoxypinoresinol
Catalog No.:BCN2161
CAS No.:81426-14-4
- Sanggenone D
Catalog No.:BCN1194
CAS No.:81422-93-7
- Cabergoline
Catalog No.:BCC5276
CAS No.:81409-90-7
- Canusesnol A
Catalog No.:BCN4350
CAS No.:816456-90-3
- 3-Dehydro-15-deoxoeucosterol
Catalog No.:BCN4351
CAS No.:81678-46-8
- 18-Beta-hydroxy-3-epi-alpha-yohimbine
Catalog No.:BCN3518
CAS No.:81703-06-2
- Rehmannioside A
Catalog No.:BCN2885
CAS No.:81720-05-0
- Rehmannioside B
Catalog No.:BCN8468
CAS No.:81720-06-1
- Rehmannioside C
Catalog No.:BCN8183
CAS No.:81720-07-2
- Rhmannioside D
Catalog No.:BCN2362
CAS No.:81720-08-3
- PSI-6130
Catalog No.:BCC1870
CAS No.:817204-33-4
- Bambuterol
Catalog No.:BCC5431
CAS No.:81732-65-2
- Praeruptorin B
Catalog No.:BCN4988
CAS No.:81740-07-0
- Methimepip dihydrobromide
Catalog No.:BCC7524
CAS No.:817636-54-7
- Quinocetone
Catalog No.:BCC9133
CAS No.:81810-66-4
The Daniel K. Inouye College of Pharmacy Scripts: Poha Berry (Physalis peruviana) with Potential Anti-inflammatory and Cancer Prevention Activities.[Pubmed:27920947]
Hawaii J Med Public Health. 2016 Nov;75(11):353-359.
The Daniel K. Inouye College of Pharmacy, during a historic event in Spring 2016, graduated the first two students in the Pacific region to earn a PhD in pharmaceutical sciences at the University of Hawai'i at Hilo. The college offers PhD programs in these five disciplines: Cancer Biology, Medicinal Chemistry, Pharmaceutics, Pharmacognosy, and Pharmacology. One of the Pharmacognosy dissertations focused on plant-derived natural products with potential anti-inflammatory and cancer chemopreventive activities. Physalis peruviana (Pp) L. originated in tropical South America. It has become naturalized and is found readily on the Island of Hawai'i. The edible fruits are commonly known as cape gooseberry or poha in Hawai'i. In part of our study, three new withanolides, physaperuvin G (1), physaperuvins I-J (2-3), along with four known withanolides, namely, 4beta-hydroxywithanolide E (4), Withaperuvin C (5), and physalactone (6), coagulin (7) were isolated from the aerial parts of P. peruviana. In addition, two known compounds, phyperunolide F (8), and withanolide S (9), were isolated and identified from the poha berry fruits. The structures and absolute stereochemistry of new compounds from poha were elucidated by several spectroscopy methods: Nuclear Magnetic Resonance (NMR) spectroscopy, X-ray diffraction, and mass spectrometry analyses. All isolated poha compounds (aerial parts and fruits) were evaluated for their anti-inflammatory activity with lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells, and tumor necrosis factor alpha (TNF-alpha)-activated nuclear factor-kappa B (NF-kappaB) with transfected human embryonic kidney cells 293. Most of the isolated natural compounds showed activity with these assays. Additional studies were performed with models of colon cancer. Specifically, 4beta-hydroxywithanolide E (4HWE) inhibited the growth of colon cancer monolayer and spheroid cultures. The compound induced cell cycle arrest at low concentrations and apoptosis at higher concentrations. These data suggest the ingestion of poha berries may have some effect on the prevalence of colon cancer. Additionally, poha isolates compounds were evaluated for their growth inhibitory effects with U251MG glioblastoma and MDA-MB-231 breast cancer cells that harbor aberrantly-active signal transducer and activation of transcription 3 (STAT3), compared to normal NIH-3T3 mouse fibroblasts. This work has led to the filing of three provisional patents with the University of Hawai'i Office of Technology Transfer and Economic Development.


