VomicineCAS# 125-15-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125-15-5 | SDF | Download SDF |
| PubChem ID | 101595 | Appearance | Powder |
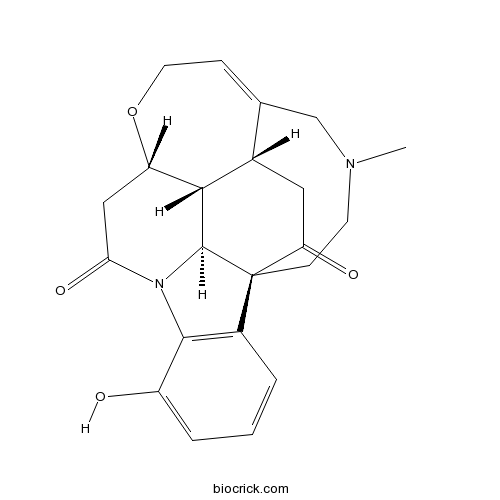
| Formula | C22H24N2O4 | M.Wt | 380.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Strychnicine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CN1CCC23C4C5C(CC2=O)C(=CCOC5CC(=O)N4C6=C3C=CC=C6O)C1 | ||
| Standard InChIKey | ZMTYENXGROJCEA-LNKPQSDASA-N | ||
| Standard InChI | InChI=1S/C22H24N2O4/c1-23-7-6-22-14-3-2-4-15(25)20(14)24-18(27)10-16-19(21(22)24)13(9-17(22)26)12(11-23)5-8-28-16/h2-5,13,16,19,21,25H,6-11H2,1H3/t13-,16-,19-,21-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vomicine is a natural product from Strychnos nux-vomica, the S. nux-vomica extracts show antihyperglycemic activity in experimental animals. |
| In vitro | New antimalarial and cytotoxic sungucine derivatives from Strychnos icaja roots.[Pubmed: 10821054]Planta Med. 2000 Apr;66(3):262-9.
Antiplasmodial alkaloids from the stem bark of Strychnos malacoclados.[Pubmed: 22193980 ]Planta Med. 2012 Mar;78(4):377-82.From the stem bark of Strychnos malacoclados, one new bisindole alkaloid, 3-hydroxylongicaudatine Y (1), was isolated along with the known alkaloids Vomicine (2), bisnordihydrotoxiferine (3), divarine (4), longicaudatine (5), longicaudatine Y (6), and longicaudatine F (7). |
| In vivo | Strychnos nux-vomica seeds: Pharmacognostical standardization, extraction, and antidiabetic activity.[Pubmed: 22707864 ]J Ayurveda Integr Med. 2012 Apr;3(2):80-4.Strychnos nux-vomica, commonly known as kuchla, contains strychnine and brucine as main constituents. Minor alkaloids present in the seeds are protostrychnine, Vomicine, n-oxystrychnine, pseudostrychnine, isostrychnine, chlorogenic acid, and a glycoside. Seeds are used traditionally to treat diabetes, asthma, aphrodisiac and to improve appetite.The present study was aimed to evaluate the various pharmacognostical characters and antidiabetic activity of S. nux-vomica seed. |

Vomicine Dilution Calculator

Vomicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6288 mL | 13.1441 mL | 26.2881 mL | 52.5762 mL | 65.7203 mL |
| 5 mM | 0.5258 mL | 2.6288 mL | 5.2576 mL | 10.5152 mL | 13.1441 mL |
| 10 mM | 0.2629 mL | 1.3144 mL | 2.6288 mL | 5.2576 mL | 6.572 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0515 mL | 1.3144 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2629 mL | 0.5258 mL | 0.6572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isobornyl acetate
Catalog No.:BCN8296
CAS No.:125-12-2
- Prednisone 21-acetate
Catalog No.:BCC9128
CAS No.:125-10-0
- Ajugamarin L2
Catalog No.:BCN3662
CAS No.:124961-67-7
- Ajugacumbin A
Catalog No.:BCN3657
CAS No.:124961-66-6
- Raddeanoside R8
Catalog No.:BCN6555
CAS No.:124961-61-1
- Tectoroside
Catalog No.:BCN6672
CAS No.:124960-89-0
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- (R)-(+)-Tolterodine
Catalog No.:BCC4054
CAS No.:124937-51-5
- Benzoyl leuco methylene blue
Catalog No.:BCC8862
CAS No.:1249-97-4
- Isoaltholactone
Catalog No.:BCN4826
CAS No.:124868-11-7
- 5-Dehydroxyparatocarpin K
Catalog No.:BCN4017
CAS No.:124858-37-3
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Primidone
Catalog No.:BCC4930
CAS No.:125-33-7
- Usnic acid
Catalog No.:BCC8264
CAS No.:125-46-2
- Aminoglutethimide
Catalog No.:BCC4368
CAS No.:125-84-8
- 8-Prenylluteone
Catalog No.:BCN4771
CAS No.:125002-91-7
- Fmoc-D-Ala-OPfp
Catalog No.:BCC3037
CAS No.:125043-04-1
- 8-(3-Ethoxy-2-hydroxy-3-methylbutyl)-7-methoxycoumarin
Catalog No.:BCN1594
CAS No.:125072-68-6
- Epinortrachelogenin
Catalog No.:BCN3719
CAS No.:125072-69-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- 26-Nor-8-oxo-alpha-onocerin
Catalog No.:BCN6131
CAS No.:125124-68-7
- 1-(4-methoxyphenyl)-2-methylpropan-1-one
Catalog No.:BCN8163
CAS No.:2040-20-2
- Rosthornin A
Catalog No.:BCN6132
CAS No.:125164-55-8
- Vibralactone D
Catalog No.:BCN6747
CAS No.:1251748-32-9
Antiplasmodial alkaloids from the stem bark of Strychnos malacoclados.[Pubmed:22193980]
Planta Med. 2012 Mar;78(4):377-82.
From the stem bark of Strychnos malacoclados, one new bisindole alkaloid, 3-hydroxylongicaudatine Y (1), was isolated along with the known alkaloids Vomicine (2), bisnordihydrotoxiferine (3), divarine (4), longicaudatine (5), longicaudatine Y (6), and longicaudatine F (7). All the compounds were tested for their antimalarial activity against the chloroquine-sensitive 3D7 and -resistant W2 strains of Plasmodium falciparum. Longicaudatine was the most active compound with IC(5)(0) values of 0.682 and 0.573 microM, respectively. The activity of compounds 1, 3, 4, 6, and 7 against the two strains ranged from 1.191 to 6.220 microM and 0.573 to 21.848 microM, respectively. Vomicine (2), the only monomer isolated, was inactive. The alkaloids of the longicaudatine-type ( 1, 5-7) were more active than those of the caracurine-type (3- 4). The presence of the ether bridge in the molecule seems to increase the antiplasmodial activity. Compounds 1, 5, and 7 were tested against the WI-38 human fibroblast cell line. Longicaudatine was the most cytotoxic compound with an IC(5)(0) of 2.721 microM. Longicaudatine F was 40-46 times more active against the two strains of P. falciparum than against the human fibroblasts and was thus considered as the more selective alkaloid. The structures of the compounds were determined based on the analysis of their spectral data.
Strychnos nux-vomica seeds: Pharmacognostical standardization, extraction, and antidiabetic activity.[Pubmed:22707864]
J Ayurveda Integr Med. 2012 Apr;3(2):80-4.
BACKGROUND: Strychnos nux-vomica, commonly known as kuchla, contains strychnine and brucine as main constituents. Minor alkaloids present in the seeds are protostrychnine, Vomicine, n-oxystrychnine, pseudostrychnine, isostrychnine, chlorogenic acid, and a glycoside. Seeds are used traditionally to treat diabetes, asthma, aphrodisiac and to improve appetite. OBJECTIVE: The present study was aimed to evaluate the various pharmacognostical characters and antidiabetic activity of S. nux-vomica seed. MATERIALS AND METHODS: Pharmacognostical characters were performed as per the WHO guideline. Extraction was carried out in petroleum ether, chloroform, alcohol, hydroalcoholic, aqueous, and phytochemical constituents present in extracts were detected by different chemical tests. Among these extracts hydroalcoholic, aqueous extracts were evaluated for antidiabetic activity on the basis of extractive yield and phytoconstituents, in alloxan-induced diabetic rats using gliclazide as standard. RESULTS: Various analytical values of S. nux-vomica extract were established. Phytoconstituents present in S. nux-vomica extracts were detected. CONCLUSION: S. nux-vomica extracts show antihyperglycemic activity in experimental animals.
New antimalarial and cytotoxic sungucine derivatives from Strychnos icaja roots.[Pubmed:10821054]
Planta Med. 2000 Apr;66(3):262-9.
Reinvestigation of Strychnos icaja Baillon resulted in the isolation of Vomicine, isostrychnine and of three new sungucine derivatives, named isosungucine (8), 18-hydroxy-sungucine (9) and 18-hydroxy-isosungucine (10). They were identified by detailed spectroscopic methods. The complete 1H- and 13C-NMR study of sungucine was also realized. Some of these compounds were highly active against Plasmodium falciparum in vitro and more particularly against the chloroquine-resistant strain. Compound 10 showed a selective antiplasmodial activity, with > 100-fold greater toxicity towards Plasmodium falciparum, relative to cultured human cancer cells (KB and HeLa lines) or fibroblasts (WI38).


