Isobornyl acetateCAS# 125-12-2 |
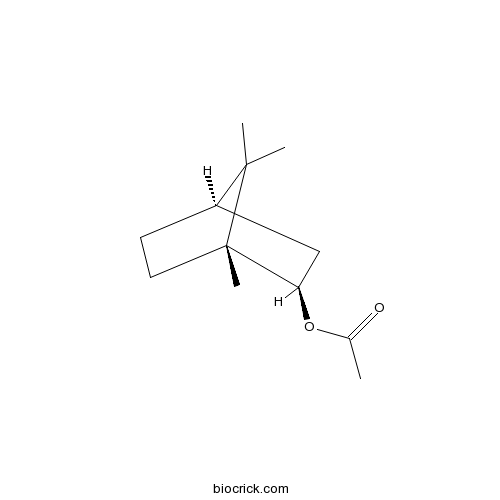
- (+)-Bornyl acetate
Catalog No.:BCN8317
CAS No.:20347-65-3
- (-)-Bornyl acetate
Catalog No.:BCN2636
CAS No.:5655-61-8
- Bornyl Acetate
Catalog No.:BCN9108
CAS No.:76-49-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125-12-2 | SDF | Download SDF |
| PubChem ID | 637531 | Appearance | Colorless liquid |
| Formula | C12H20O2 | M.Wt | 196.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Isobornyl ethanoate;Pichtosin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,2R,4R)-1,7,7-trimethyl-2-bicyclo[2.2.1]heptanyl] acetate | ||
| SMILES | CC(=O)OC1CC2CCC1(C2(C)C)C | ||
| Standard InChIKey | KGEKLUUHTZCSIP-FOGDFJRCSA-N | ||
| Standard InChI | InChI=1S/C12H20O2/c1-8(13)14-10-7-9-5-6-12(10,4)11(9,2)3/h9-10H,5-7H2,1-4H3/t9-,10-,12+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isobornyl acetate Dilution Calculator

Isobornyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0945 mL | 25.4725 mL | 50.945 mL | 101.8901 mL | 127.3626 mL |
| 5 mM | 1.0189 mL | 5.0945 mL | 10.189 mL | 20.378 mL | 25.4725 mL |
| 10 mM | 0.5095 mL | 2.5473 mL | 5.0945 mL | 10.189 mL | 12.7363 mL |
| 50 mM | 0.1019 mL | 0.5095 mL | 1.0189 mL | 2.0378 mL | 2.5473 mL |
| 100 mM | 0.0509 mL | 0.2547 mL | 0.5095 mL | 1.0189 mL | 1.2736 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Prednisone 21-acetate
Catalog No.:BCC9128
CAS No.:125-10-0
- Ajugamarin L2
Catalog No.:BCN3662
CAS No.:124961-67-7
- Ajugacumbin A
Catalog No.:BCN3657
CAS No.:124961-66-6
- Raddeanoside R8
Catalog No.:BCN6555
CAS No.:124961-61-1
- Tectoroside
Catalog No.:BCN6672
CAS No.:124960-89-0
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- (R)-(+)-Tolterodine
Catalog No.:BCC4054
CAS No.:124937-51-5
- Benzoyl leuco methylene blue
Catalog No.:BCC8862
CAS No.:1249-97-4
- Isoaltholactone
Catalog No.:BCN4826
CAS No.:124868-11-7
- 5-Dehydroxyparatocarpin K
Catalog No.:BCN4017
CAS No.:124858-37-3
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Valacyclovir hydrochloride
Catalog No.:BCC4051
CAS No.:124832-27-5
- Vomicine
Catalog No.:BCN6735
CAS No.:125-15-5
- Primidone
Catalog No.:BCC4930
CAS No.:125-33-7
- Usnic acid
Catalog No.:BCC8264
CAS No.:125-46-2
- Aminoglutethimide
Catalog No.:BCC4368
CAS No.:125-84-8
- 8-Prenylluteone
Catalog No.:BCN4771
CAS No.:125002-91-7
- Fmoc-D-Ala-OPfp
Catalog No.:BCC3037
CAS No.:125043-04-1
- 8-(3-Ethoxy-2-hydroxy-3-methylbutyl)-7-methoxycoumarin
Catalog No.:BCN1594
CAS No.:125072-68-6
- Epinortrachelogenin
Catalog No.:BCN3719
CAS No.:125072-69-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- 26-Nor-8-oxo-alpha-onocerin
Catalog No.:BCN6131
CAS No.:125124-68-7
- 1-(4-methoxyphenyl)-2-methylpropan-1-one
Catalog No.:BCN8163
CAS No.:2040-20-2
- Rosthornin A
Catalog No.:BCN6132
CAS No.:125164-55-8
Oral 1-Generation Rat Reproduction Study of Isobornyl Acetate: An Evaluation Through Sexual Maturity in the F1 Generation.[Pubmed:28481133]
Int J Toxicol. 2017 May/Jun;36(3):252-259.
Reproductive toxicity of Isobornyl acetate (IA), a widely used fragrance ingredient, was investigated in a 1-generation reproduction study in which 25 Crl: CD (Sprague-Dawley) rats/sex/group were gavaged with dosages of 0 (corn oil vehicle), 30, 100, or 300 mg/kg/d during premating, mating, gestation, and lactation. After weaning, 25 F1 generation pups/sex/dosage group were randomly selected for evaluation until sexual maturity. The following parameters were evaluated in P generation males and females: viability, clinical signs, body weights, feed consumption, mating and fertility, organ weights, gross and microscopic observations, sperm assessments (motility and concentration), natural delivery and litter observations, and ovarian follicle counts. In F1 generation pups, viability, body weights, sexual maturation, anogenital distance (days 1 and 22 postpartum), nipple eruption (day 12 postpartum), and gross necropsy observations were recorded. Isobornyl acetate did not adversely affect any of the investigated parameters. Based on the results of this investigation, the no observable adverse effect level (NOAEL) for toxicity of IA is considered to be 300 mg/kg/d. Increased incidences of excess salivation occurred in P generation male and female rats at 100 and/or 300 mg/kg/d throughout the dosage period, and low incidences of urine-stained abdominal fur were seen in females at 300 mg/kg/d during the gestation period. These clinical signs were not considered as adverse effects of IA administration. Thus, the NOAEL for reproductive toxicity in the P generation rats and the NOAEL for viability and growth of the F1 generation offspring is considered to be >/=300 mg/kg/d.
Chromatographic Fingerprints Combined with Chemometric Methods Reveal the Chemical Features of Authentic Radix Polygalae.[Pubmed:27743444]
J AOAC Int. 2017 Jan 1;100(1):30-37.
GC-MS fingerprints of Radix Polygalae (RP) were measured for deliberately collected samples. A total of 88 volatile components were identified and quantified by subwindow factor analysis, heuristic evolving latent projection, and retention index. Next, an efficient discrimination model based on partial least-squares (PLS) discriminant analysis (DA) was developed to distinguish the superior RP samples from the inferior ones, and the reliability and predictive ability of the model was evaluated by cross-validation and permutation tests. Furthermore, four components (1-octanol, shyobunone, Isobornyl acetate, and alpha-asarone) were screened by coefficient beta of PLS-DA. They represented the important chemical features of authentic RP and could be applied to the accurate discrimination and QC of RP in the future. Our results suggest that chromatographic fingerprints coupled with chemometric methods provide an effective and convenient strategy for QC of RP and are helpful for revealing the chemical features of a complex analytical sample.
Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents.[Pubmed:27586050]
J Agric Food Chem. 2016 Sep 28;64(38):7156-70.
Essential oils were obtained by hydrodistillation of the umbels+seeds and stems of Ferula akitschkensis (FAEOu/s and FAEOstm, respectively) and analyzed by gas chromatography and gas chromatography-mass spectrometry. Fifty-two compounds were identified in FAEOu/s; the primary components were sabinene, alpha-pinene, beta-pinene, terpinen-4-ol, eremophilene, and 2-himachalen-7-ol, whereas the primary components of FAEOstm were myristicin and geranylacetone. FAEOu/s, beta-pinene, sabinene, gamma-terpinene, geranylacetone, Isobornyl acetate, and (E)-2-nonenal stimulated [Ca(2+)]i mobilization in human neutrophils, with the most potent being geranylacetone (EC50 = 7.6 +/- 1.9 muM) and Isobornyl acetate 6.4 +/- 1.7 (EC50 = 7.6 +/- 1.9 muM). In addition, treatment of neutrophils with beta-pinene, sabinene, gamma-terpinene, geranylacetone, and Isobornyl acetate desensitized the cells to N-formyl-Met-Leu-Phe (fMLF)- and interleukin-8 (IL-8)-induced [Ca(2+)]i flux and inhibited fMLF-induced chemotaxis. The effects of beta-pinene, sabinene, gamma-terpinene, geranylacetone, and Isobornyl acetate on neutrophil [Ca(2+)]i flux were inhibited by transient receptor potential (TRP) channel blockers. Furthermore, the most potent compound, geranylacetone, activated Ca(2+) influx in TRPV1-transfected HEK293 cells. In contrast, myristicin inhibited neutrophil [Ca(2+)]i flux stimulated by fMLF and IL-8 and inhibited capsaicin-induced Ca(2+) influx in TRPV1-transfected HEK293 cells. These findings, as well as pharmacophore modeling of TRP agonists, suggest that geranylacetone is a TRPV1 agonist, whereas myristicin is a TRPV1 antagonist. Thus, at least part of the medicinal properties of Ferula essential oils may be due to modulatory effects on TRP channels.
RIFM fragrance ingredient safety assessment, isobornyl isovalerate, CAS registry number 7779-73-9.[Pubmed:27815161]
Food Chem Toxicol. 2017 Dec;110 Suppl 1:S1-S8.
This material was evaluated for genotoxicity, repeated dose toxicity, developmental toxicity, reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin sensitization potential, as well as, environmental safety. Data from the suitable read across analog Isobornyl acetate (CAS # 125-12-2) show that this material is not genotoxic, provided a MOE > 100 for the repeated dose, developmental and reproductive endpoints, and does not have skin sensitization potential. The local respiratory toxicity endpoint was completed using the TTC (threshold of Toxicological Concern) for a Cramer Class II material (0.47 mg/day). The phototoxicity/photoallergenicity endpoint was completed based on suitable UV spectra. The environmental endpoint was completed as described in the RIFM Framework.
Salvia broussonetii Benth.: aroma profile and micromorphological analysis.[Pubmed:29072096]
Nat Prod Res. 2018 Jul;32(14):1660-1668.
The volatile profiles (VOC) and the essential oil (EO) composition from the aerial parts of Salvia broussonetii were analysed. Sesquiterpene hydrocarbons dominate the VOCs from leaves (95.7%) and flowers (67.6%), followed by monoterpene hydrocarbons (2.6 and 29.7%, respectively). The main common compounds are germacrene D, beta-bourbonene, alpha-pinene, alpha-copaene and alpha-gurjunene, even if with divergent relative abundances. In the leaf EOs the sesquiterpenes prevail, even if not overwhelmingly (about 50.0%), followed by monoterpenes (23.0-35.0%) and by minor fractions of diterpene hydrocarbons and non-terpene derivates. The most abundant common compounds across the two sampling periods are alpha-pinene, beta-pinene, Isobornyl acetate, alpha-gurjenene, germacrene D and bifloratriene. A morphological characterisation of the trichomes responsible for the productivity in terpenes was also performed. Four different morphotypes were observed on the above ground organs of S. brussonetii: peltates and capitates of type II and III resulted the only producers of volatile substances.
Chemical composition of the essential oil of Croton bonplandianus from India.[Pubmed:24689307]
Nat Prod Commun. 2014 Feb;9(2):269-70.
The essential oil obtained from the aerial parts of Croton bonplandianus Baill. was analyzed by gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS). A total of 37 compounds have been identified, representing 96.2% of the total oil. The main constituents were identified as beta-caryophyllene (16.7%), germacrene D (14.7%), borneol (8.3%), Z-beta-damascenone (6.(%), Isobornyl acetate (6.2%), alpha-humulene (6.1%), germacrene A (5.2%) and caryophyllene oxide (4.5%). The oil was rich in sesquiterpene hydrocarbons (60.1%).
Variation of Chemical Composition in Flowers and Leaves Essential Oils Among Natural Population of Tunisian Glebionis coronaria (L.) Tzvelev (Asteraceae).[Pubmed:27451137]
Chem Biodivers. 2016 Oct;13(10):1251-1261.
The aim of this study was to assess the percentage and constituents variations in flowers and leaves essential oil of three Glebionis coronaria (L.) Tzvelev population, growing wildly in three different ecotypes (Utique, M'saken, and Sahara Lektar) in Tunisia. The chemical compositions of these essential oils were analyzed by the GC and GC/MS systems. Qualitative and quantitative differences were recorded between essential oils extracted from plants collected from the three geographical provinces and between organs of the same plant (leaves and flowers). In fact, 161 components representing 87.2 - 96.5% of the whole oils were identified. Myrcene (3.2 - 35.7%), (Z)-beta-ocimene (0.6 - 23.0%), camphor (0.6 - 17.2%), cis-chrysanthenol (0 - 6.9%), cis-chrysanthenyl acetate (1.1 - 17.9%), Isobornyl acetate (1.6 - 3.5%), (E)-beta-farnesene (0 - 6.0%), germacrene D (0 - 8.7%), and (E,E)-alpha-farnesene (0.7 - 12.4%) were the predominant components in the oils. These major constituents occur in different amounts depending on the organs (leaves or flowers) and the geographical origin of the plant. The chemotaxonomic usefulness of these data was discussed according to results of principal component analysis (PCA). The scores, together with the loadings, revealed a different chemical pattern for each population.
Volatile organic compounds obtained by in vitro callus cultivation of Plectranthus ornatus Codd. (Lamiaceae).[Pubmed:24064448]
Molecules. 2013 Aug 26;18(9):10320-33.
Plectranthus spp (Lamiaceae) are plants of economic importance because they are sources of aromatic essential oils and are also cultivated and several species of this genus are used as folk medicines. This paper describes the effects of different concentrations of the 2,4-dichlorophenoxyacetic acid (2,4-D) and 1-naphthaleneacetic acid (NAA) on the induction of callus from nodal segments of Plectranthus ornatus Codd and in the production of volatile organic compounds (monoterpenes and sesquiterpenes). The 20 and 40 day calli were subjected to solid phase micro extraction (HS-SPME) and submitted to GCMS analysis. Variations in VOCs between the samples were observed and, a direct relationship was observed between of the major constituent detected (alpha-terpinyl acetate) and the monoterpenes alpha-thujene, alpha-pinene, beta-pinene, camphene, sabinene and alpha-limonene that were present in the volatile fractions. Besides alpha-terpinyl acetate, Isobornyl acetate and alpha-limonene were also major constituents. Variations were observed in VOCs in the analyzed periods. The best cultivation media for the production of VOCs was found to be MS0 (control). Moderate success was achieved by treatment with 2.68 microM and 5:37 microM NAA (Group 2). With 2,4-D (9.0 microM), only the presence of alpha-terpinyl acetate and isocumene were detected and, with 2.26 microM of 2,4-D was produced mainly alpha-terpinyl acetate, alpha-thujene and beta-caryophyllene (16.2%). The VOC profiles present in P. ornatus were interpreted using PCA and HCA. The results permitted us to determine the best cultivation media for VOC production and, the PCA and HCA analysis allowed us to recognize four groups among the different treatments from the compounds identified in this set of treatments.


