VellosimineCAS# 6874-98-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6874-98-2 | SDF | Download SDF |
| PubChem ID | 5281961 | Appearance | Powder |
| Formula | C19H20N2O | M.Wt | 292.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
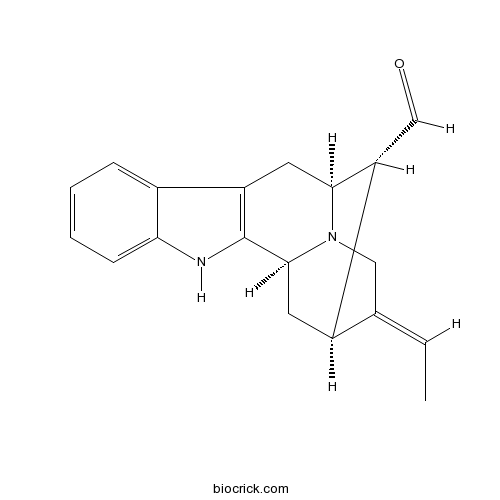
| SMILES | CC=C1CN2C3CC1C(C2CC4=C3NC5=CC=CC=C45)C=O | ||
| Standard InChIKey | MHASSCPGKAMILD-PQKPIAESSA-N | ||
| Standard InChI | InChI=1S/C19H20N2O/c1-2-11-9-21-17-8-14-12-5-3-4-6-16(12)20-19(14)18(21)7-13(11)15(17)10-22/h2-6,10,13,15,17-18,20H,7-9H2,1H3/b11-2-/t13-,15-,17+,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Org Lett. 2000 Jul 13;2(14):2057-9.General approach for the synthesis of sarpagine/ajmaline indole alkaloids. Stereospecific total synthesis of the sarpagine alkaloid (+)-vellosimine.[Pubmed: 10891229] Angew Chem Int Ed Engl. 2015 Jan 2;54(1):315-7.Enantioselective, protecting-group-free total synthesis of sarpagine alkaloids--a generalized approach.[Pubmed: 25346454]
|

Vellosimine Dilution Calculator

Vellosimine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.42 mL | 17.0999 mL | 34.1997 mL | 68.3995 mL | 85.4993 mL |
| 5 mM | 0.684 mL | 3.42 mL | 6.8399 mL | 13.6799 mL | 17.0999 mL |
| 10 mM | 0.342 mL | 1.71 mL | 3.42 mL | 6.8399 mL | 8.5499 mL |
| 50 mM | 0.0684 mL | 0.342 mL | 0.684 mL | 1.368 mL | 1.71 mL |
| 100 mM | 0.0342 mL | 0.171 mL | 0.342 mL | 0.684 mL | 0.855 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arborine
Catalog No.:BCN7480
CAS No.:6873-15-0
- Phellodendrine
Catalog No.:BCN5933
CAS No.:6873-13-8
- Epiberberine
Catalog No.:BCN5387
CAS No.:6873-09-2
- Xanthoplanine
Catalog No.:BCN4246
CAS No.:6872-88-4
- Arteanoflavone
Catalog No.:BCN6824
CAS No.:68710-17-8
- (-)-Lotusine
Catalog No.:BCN8443
CAS No.:6871-67-6
- Echitamine
Catalog No.:BCN4245
CAS No.:6871-44-9
- Asimilobine
Catalog No.:BCN7076
CAS No.:6871-21-2
- 13,18-Dehydroglaucarubinone
Catalog No.:BCN7957
CAS No.:68703-94-6
- 11β,17α-Dihydroxy-6α-methylpregna-1,4-diene-3,20-dione
Catalog No.:BCC8434
CAS No.:6870-94-6
- Jacobine
Catalog No.:BCN2087
CAS No.:6870-67-3
- Retronecine N-oxide
Catalog No.:BCN2035
CAS No.:6870-33-3
- (±)-Pinocembrin
Catalog No.:BCN3537
CAS No.:68745-38-0
- Loxoprofen
Catalog No.:BCC5191
CAS No.:68767-14-6
- Corynoxine
Catalog No.:BCN2364
CAS No.:6877-32-3
- 12-Hydroxy-2,3-dihydroeuparin
Catalog No.:BCN8115
CAS No.:68776-42-1
- 1beta-Hydroxyalantolactone
Catalog No.:BCN3508
CAS No.:68776-47-6
- Triclabendazole
Catalog No.:BCC4742
CAS No.:68786-66-3
- Tuberstemonine
Catalog No.:BCN4986
CAS No.:6879-01-2
- Dehydroeburicoic acid
Catalog No.:BCN3646
CAS No.:6879-05-6
- Dipotassium glycyrrhizinate
Catalog No.:BCN8487
CAS No.:68797-35-3
- 4-Oxobedfordiaic acid
Catalog No.:BCN4247
CAS No.:68799-38-2
- 6-Acetyl-2,2-dimethylchroman-4-one
Catalog No.:BCN4248
CAS No.:68799-41-7
- Norfluorocurarine
Catalog No.:BCN4811
CAS No.:6880-54-2
General approach for the synthesis of sarpagine/ajmaline indole alkaloids. Stereospecific total synthesis of the sarpagine alkaloid (+)-vellosimine.[Pubmed:10891229]
Org Lett. 2000 Jul 13;2(14):2057-9.
[reaction: see text] (+)-Vellosimine has been synthesized enantiospecifically in 27% overall yield from commercially available D-(+)-tryptophan methyl ester via the asymmetric Pictet-Spengler reaction and a stereocontrolled intramolecular palladium-coupling reaction as key steps.
Enantioselective, protecting-group-free total synthesis of sarpagine alkaloids--a generalized approach.[Pubmed:25346454]
Angew Chem Int Ed Engl. 2015 Jan 2;54(1):315-7.
A generalized synthetic access to sarpagine alkaloids through a joint synthetic sequence has been accomplished. Its applicability is showcased by the enantioselective total syntheses of Vellosimine (1), N-methylVellosimine (3), and 10-methoxyVellosimine (8). The synthetic sequence is concise (eight steps) from known compound 13, and requires no protecting groups. The indole heterocycle was introduced in the last step. This strategy allows access to sarpagine alkaloids through a shared synthetic route leading to precursor 10, which we term "privileged intermediate". Starting from this intermediate, all sarpagine alkaloids can be synthesized using phenylhydrazines with different substitution patterns (15-17). Our approach brings about the advantage, that synthesis optimization only needs to be performed once for many natural products. The key features of the synthesis are a [5+2]-cycloaddition and a ring enlargement.


