Vanillyl alcoholCAS# 498-00-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 498-00-0 | SDF | Download SDF |
| PubChem ID | 62348 | Appearance | Powder |
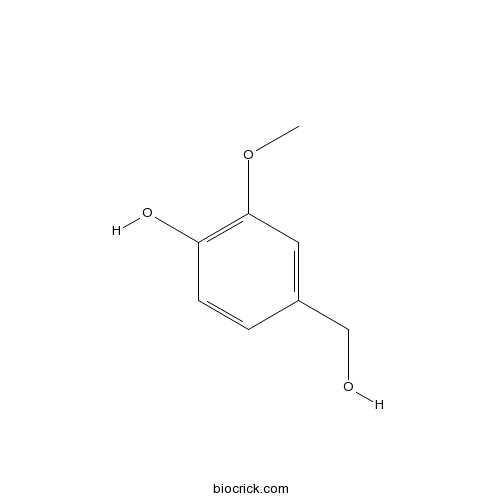
| Formula | C8H10O3 | M.Wt | 154.17 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (810.85 mM; Need ultrasonic) | ||
| Chemical Name | 4-(hydroxymethyl)-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)CO)O | ||
| Standard InChIKey | ZENOXNGFMSCLLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H10O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-4,9-10H,5H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vanillyl alcohol possesses anti-angiogenic, anticonvulsive, anti-inflammatory, anti-oxidant, neuroprotective, and anti-nociceptive activities. Vanillyl alcohol can effectively inhibit the cytotoxicity and improved cell viability in 1-methyl-4-phenylpyridinium (MPP+)-induced MN9D dopaminergic cells, it also can attenuate the elevation of reactive oxygen species (ROS) levels, decrease in the Bax/Bcl-2 ratio and poly (ADP-ribose) polymerase proteolysis. |
| Targets | ROS | PARP | Bcl-2/Bax |
| In vitro | Specificity of maltase to maltose in three different directions of reaction: hydrolytic, vanillyl alcohol glucoside and vanillyl alcohol isomaltoside synthesis.[Pubmed: 22927369]Biotechnol Prog. 2012 Nov-Dec;28(6):1450-6.Vanillyl alcohol glucoside is very attractive molecule due to its very powerful physiological activity. Ultrasonographic Imaging and Anti-inflammatory Therapy of Muscle and Tendon Injuries Using Polymer Nanoparticles.[Pubmed: 28744328 ]Theranostics. 2017 Jun 24;7(9):2463-2476.Ultrasonography is a reliable diagnostic modality for muscle and tendon injuries, but it has been challenging to find right diagnosis of minor musculoskeletal injuries by conventional ultrasonographic imaging. |
| Cell Research | Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells.[Pubmed: 21705974 ]Molecules, 2011, 16(7):5349-61.Gastrodia elata Blume (GE) has long been used in oriental countries as a traditional herbal medicine to relieve symptoms associated with neurological ailments such as vertigo, general paralysis and epilepsy. |

Vanillyl alcohol Dilution Calculator

Vanillyl alcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4863 mL | 32.4317 mL | 64.8635 mL | 129.7269 mL | 162.1587 mL |
| 5 mM | 1.2973 mL | 6.4863 mL | 12.9727 mL | 25.9454 mL | 32.4317 mL |
| 10 mM | 0.6486 mL | 3.2432 mL | 6.4863 mL | 12.9727 mL | 16.2159 mL |
| 50 mM | 0.1297 mL | 0.6486 mL | 1.2973 mL | 2.5945 mL | 3.2432 mL |
| 100 mM | 0.0649 mL | 0.3243 mL | 0.6486 mL | 1.2973 mL | 1.6216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- Stiripentol
Catalog No.:BCC3977
CAS No.:49763-96-4
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
- Ethyl Nipecotate
Catalog No.:BCC3272
CAS No.:5006-62-2
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells.[Pubmed:21705974]
Molecules. 2011 Jun 24;16(7):5349-61.
Gastrodia elata Blume (GE) has long been used in oriental countries as a traditional herbal medicine to relieve symptoms associated with neurological ailments such as vertigo, general paralysis and epilepsy. In this study, we have investigated the effects of GE extracts and its major bioactive components on 1-methyl-4-phenylpyridinium (MPP+)-treated MN9D dopaminergic cells, a classic in vitro model for Parkinson's disease (PD). We found that Vanillyl alcohol effectively inhibited the cytotoxicity and improved cell viability in MPP+-induced MN9D dopaminergic cells. The underlying mechanisms of Vanillyl alcohol action were also studied. Vanillyl alcohol attenuated the elevation of reactive oxygen species (ROS) levels, decreased in the Bax/Bcl-2 ratio and poly (ADP-ribose) polymerase proteolysis. These results indicate that Vanillyl alcohol protected dopaminergic MN9D cells against MPP+-induced apoptosis by relieving oxidative stress and modulating the apoptotic process and is therefore a potential candidate for treatment of neurodegenerative diseases such as Parkinson's disease.
Specificity of maltase to maltose in three different directions of reaction: hydrolytic, vanillyl alcohol glucoside and vanillyl alcohol isomaltoside synthesis.[Pubmed:22927369]
Biotechnol Prog. 2012 Nov-Dec;28(6):1450-6.
Vanillyl alcohol glucoside is very attractive molecule due to its very powerful physiological activity. In this article, a detailed kinetic study of transglucosylation of Vanillyl alcohol was performed. It was demonstrated that this reaction is very efficient (selectivity factor is 149) and occurred by a ping-pong mechanism with inhibition by glucose acceptor. At low concentration of Vanillyl alcohol one additional transglucosylation product was detected. Its structure was determined to be alpha-isomaltoside of Vanillyl alcohol, indicating that Vanillyl alcohol glucoside is a product of the first transglucosylation reaction and a substrate for second, so the whole reaction mechanism was proposed. It was demonstrated that the rate of isomaltoside synthesis is two orders of magnitude smaller than glucoside synthesis, and that maltase has interestingly high K(m) value to maltose when Vanillyl alcohol glucoside is second transglucosylation substrate.


