StiripentolAn LDH inhibitor CAS# 49763-96-4 |
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 49763-96-4 | SDF | Download SDF |
| PubChem ID | 5311454 | Appearance | Powder |
| Formula | C14H18O3 | M.Wt | 234.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (640.23 mM; Need ultrasonic and warming) | ||
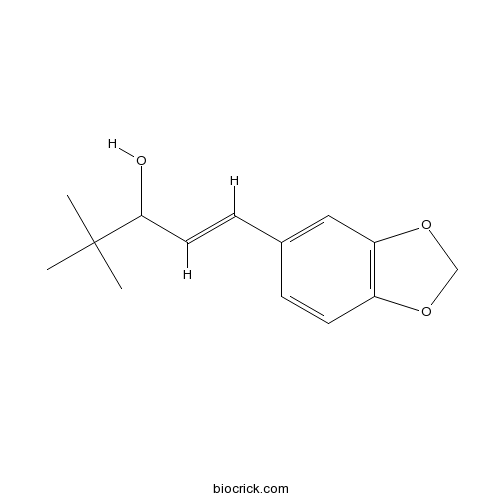
| Chemical Name | (E)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol | ||
| SMILES | CC(C)(C)C(C=CC1=CC2=C(C=C1)OCO2)O | ||
| Standard InChIKey | IBLNKMRFIPWSOY-FNORWQNLSA-N | ||
| Standard InChI | InChI=1S/C14H18O3/c1-14(2,3)13(15)7-5-10-4-6-11-12(8-10)17-9-16-11/h4-8,13,15H,9H2,1-3H3/b7-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Stiripentol Dilution Calculator

Stiripentol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2682 mL | 21.3411 mL | 42.6821 mL | 85.3643 mL | 106.7054 mL |
| 5 mM | 0.8536 mL | 4.2682 mL | 8.5364 mL | 17.0729 mL | 21.3411 mL |
| 10 mM | 0.4268 mL | 2.1341 mL | 4.2682 mL | 8.5364 mL | 10.6705 mL |
| 50 mM | 0.0854 mL | 0.4268 mL | 0.8536 mL | 1.7073 mL | 2.1341 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.0671 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Stiripentol is an LDH inhibitor. It is a new-generation antiepileptic drug, and its chemical structure is unrelated to other antiepileptic drugs. Seizures and epileptiform activity are reduced by inhibition of the metabolic pathway via lactate dehydrogenase (LDH), which is a component of the astrocyte-neuron lactate shuttle [1].
Stiripentol is used for the treatment of Dravet syndrome, a rare form of epilepsy (26). Stiripentol (300 mg/kg ip) had a small effect on high-voltage spikes in the kainate model. Stiripentol (500 mM) inhibited the lactate-to-pyruvate conversion as well as the pyruvate-to-lactate conversion by both human LDHs. Lineweaver-Burk plots revealed that the inhibition by stiripentol was noncompetitive, suggesting that stiripentol binds to the LDHs at a different site with lactate and pyruvate. [1]
References:
[1]. Sada N, Lee S2, Katsu T et al. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015 Mar 20;347(6228):1362-7.
- H-D-Phe(4-Me)-OH
Catalog No.:BCC3271
CAS No.:49759-61-7
- Dobutamine hydrochloride
Catalog No.:BCC5391
CAS No.:49745-95-1
- Tupichinol A
Catalog No.:BCN7697
CAS No.:497142-88-8
- DC_AC50
Catalog No.:BCC6488
CAS No.:497061-48-0
- Z-Thr-NH2
Catalog No.:BCC2738
CAS No.:49705-98-8
- (-)-Praeruptorin B
Catalog No.:BCN7665
CAS No.:4970-26-7
- Arbutin
Catalog No.:BCN6307
CAS No.:497-76-7
- Adarotene
Catalog No.:BCC1328
CAS No.:496868-77-0
- Drupacine
Catalog No.:BCN7065
CAS No.:49686-57-9
- HhAntag
Catalog No.:BCC1617
CAS No.:496794-70-8
- AR-C155858
Catalog No.:BCC1367
CAS No.:496791-37-8
- Crobarbatine
Catalog No.:BCN2069
CAS No.:49679-23-4
- 11beta-Hydroxylupeol
Catalog No.:BCN7571
CAS No.:49776-92-3
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- Vanillyl alcohol
Catalog No.:BCN3832
CAS No.:498-00-0
- Acetovanillone
Catalog No.:BCN2916
CAS No.:498-02-2
- 1,6-Anhydro-β-D-glucose
Catalog No.:BCC8427
CAS No.:498-07-7
- Scopine
Catalog No.:BCN1940
CAS No.:498-45-3
- Ethyl Nipecotate
Catalog No.:BCC3272
CAS No.:5006-62-2
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
Audit of use of stiripentol in adults with Dravet syndrome.[Pubmed:27231140]
Acta Neurol Scand. 2017 Jan;135(1):73-79.
OBJECTIVES: There are very few data available in the literature on the use of Stiripentol in adults with Dravet syndrome (DS). DS cases are increasingly recognized in adulthood, and more children with DS now survive to adulthood. The aim of the study was to document the effectiveness and tolerability of Stiripentol in adults with DS. MATERIAL AND METHODS: We conducted an observational clinical audit in the epilepsy service of the National Hospital for Neurology and Neurosurgery, London (UK). RESULTS: We included 13 adult subjects with DS (eight females, five males). The responder (defined as more than 50% reduction in all seizure types) rate was 3/13 (23%) at 36 months. The following other outcomes were reported: seizure exacerbation (3/13, 23%), no change (3/13, 23%), less than 50% reduction in seizures (2/13, 15%), more than 50% reduction in generalized tonic-clonic seizures but no other seizure types (1/13, 8%), undefined response (1/13, 8%). The retention rate was 62% after 1 year and 31% after 5 years. Adverse effects were reported in 7/13 (54%): the most frequent were anorexia, weight loss, unsteadiness and tiredness. Withdrawal due to adverse effects occurred in 3/13 (23%). CONCLUSIONS: Compared with previous studies on children with DS, our results show a lower responder rate and a similar tolerability profile. Stiripentol can be effective with a good tolerability profile. Our audit is small, but supports the use of Stiripentol in adults with DS when first-line treatments are ineffective or not tolerated, in keeping with published guidelines.
Patients with dravet syndrome in the era of stiripentol: A French cohort cross-sectional study.[Pubmed:27389706]
Epilepsy Res. 2016 Sep;125:42-6.
OBJECTIVE: The aim of this study was to assess outcome and seizure response to treatment with Stiripentol (STP) associated to valproate (VPA) and clobazam (CLB), which we have used in our center since the 1990s, in patients with Dravet syndrome (DS). METHODS: We performed a cross-sectional study of all DS patients with SCN1A mutations who had at least one visit to our center in 2013. A total of 54 patients were included (32 males, 22 females), whose ages ranged from 2.5 to 22 years. RESULTS: Seizure onset ranged from 2 to 9 months (mean 5 months). Treatment started at a mean age of 7 months with valproate (VPA) as first therapy in 83% of patients. STP was prescribed in 96% at an average age of 20 months. At last follow-up (up to 22 years, median 8 years), 96% were still receiving STP, with VPA and clobazam (CLB) in 91%. Additional therapies were prescribed in 72% of patients. Most patients (96%) continued to have clonic or tonic-clonic seizures but they were brief (<5min, with last status epilepticus (SE) episode being before 4 years of age). Seizures occurred weekly (>3/month) in 38% of patients, monthly (1-3/month) in 40%, and yearly in the remaining patients. None presented with daily seizures. Seizure frequency at last visit was related to the age of treatment initiation, the age of last SE, and SCN1A mutation type. CONCLUSIONS: Triple therapy with STP, VPA, and CLB was maintained long-term by 96% of this large DS cohort because the reduced frequency and severity of seizures STP provided when added to CLB and VPA was durable. Nevertheless, only a few patients achieved seizure freedom and persisting seizures remains a concern in the majority of patients.
Add-on stiripentol elevates serum valproate levels in patients with or without concomitant topiramate therapy.[Pubmed:28081475]
Epilepsy Res. 2017 Feb;130:7-12.
OBJECTIVE: Stiripentol (STP), valproate (VPA) and topiramate (TPM) are reported to have efficacy for Dravet syndrome. In this study, we sought to elucidate the mechanisms underlying the increased serum VPA concentrations following STP adjunctive therapy in patients with Dravet syndrome. METHODS: Twenty-eight patients with Dravet syndrome (age range, 1-35 years) undergoing combination therapy with VPA and STP were included in this study. We evaluated VPA and clobazam (CLB) serum concentrations before and after add-on STP. We also investigated potential factors impacting VPA metabolism during add-on STP therapy, including add-on TPM and CYP2C9 and CYP2C19 gene polymorphisms. The effect of STP on the metabolism of concomitantly administered CLB was also investigated. RESULTS: Add-on STP was significantly associated with the serum concentration-to-dose (CD) ratio of VPA. Two patients, who were concomitantly treated with TPM, developed severe anorexia and thrombocytopenia because of marked increases in serum VPA concentrations. Further analysis revealed that the rate of increase in the VPA CD ratio was positively correlated with TPM dose. In patients not receiving TPM, STP enhanced the rate of increase in the VPA CD ratio to a significantly greater extent in CYP2C19 extensive metabolizers than in CYP2C19 poor metabolizers. Add-on STP was also associated with significant increases in CLB and N-desmethyl-CLB serum concentrations. CONCLUSION: Our findings suggest that serum VPA concentrations should be carefully monitored during STP adjunctive therapy, particularly in patients receiving concomitant TPM therapy.


