UncinatoneCAS# 99624-92-7 |
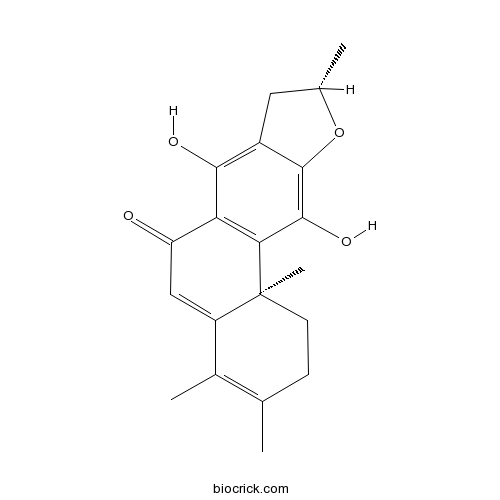
- (11Bs)-7,11-dihydroxy-3,4,9,11b-tetramethyl-1,2,8,9-tetrahydronaphtho[2,1-f][1]benzofuran-6-one
Catalog No.:BCN9161
CAS No.:
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99624-92-7 | SDF | Download SDF |
| PubChem ID | 442547 | Appearance | Orange powder |
| Formula | C20H22O4 | M.Wt | 326.39 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 1770782-39-2 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (9S,11bS)-7,11-dihydroxy-3,4,9,11b-tetramethyl-1,2,8,9-tetrahydronaphtho[2,1-f][1]benzofuran-6-one | ||
| SMILES | CC1CC2=C(C3=C(C(=C2O1)O)C4(CCC(=C(C4=CC3=O)C)C)C)O | ||
| Standard InChIKey | IQGPVLVWUUPQMQ-FVINQWEUSA-N | ||
| Standard InChI | InChI=1S/C20H22O4/c1-9-5-6-20(4)13(11(9)3)8-14(21)15-16(20)18(23)19-12(17(15)22)7-10(2)24-19/h8,10,22-23H,5-7H2,1-4H3/t10-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Uncinatone exhibits inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC50 values of 12.50 uM. 2. Uncinatone shows inhibitory activity against complement system with 50% inhibitory concentrations (IC(50)) values of 87 uM. 3. Uncinatone demonstrates cytotoxic activities against the HL-60 tumour cell line (IC50 < 20 uM). 4. Uncinatone exhibits moderate cytotoxicity, inhibits cell proliferation, and induces cell-cycle G(2)/M phase arrest. |
| Targets | NO |

Uncinatone Dilution Calculator

Uncinatone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0638 mL | 15.3191 mL | 30.6382 mL | 61.2764 mL | 76.5955 mL |
| 5 mM | 0.6128 mL | 3.0638 mL | 6.1276 mL | 12.2553 mL | 15.3191 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0638 mL | 6.1276 mL | 7.6595 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6128 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6128 mL | 0.766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Isothymonin
Catalog No.:BCN3393
CAS No.:99615-01-7
- Leucanthogenin
Catalog No.:BCN7932
CAS No.:99615-00-6
- Ondansetron
Catalog No.:BCC5043
CAS No.:99614-02-5
- Ondansetron HCl
Catalog No.:BCC2493
CAS No.:99614-01-4
- Sertaconazole nitrate
Catalog No.:BCC4716
CAS No.:99592-39-9
- Sertaconazole
Catalog No.:BCC9146
CAS No.:99592-32-2
- Neuropeptide FF
Catalog No.:BCC5983
CAS No.:99566-27-5
- K 252a
Catalog No.:BCC7152
CAS No.:99533-80-9
- L-651,582
Catalog No.:BCC7561
CAS No.:99519-84-3
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
- Kaempferol 3-O-arabinoside
Catalog No.:BCN4541
CAS No.:99882-10-7
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- Bullatantriol
Catalog No.:BCN4543
CAS No.:99933-32-1
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
A new bioactive diterpenoid from pestalotiopsis adusta, an endophytic fungus from clerodendrum canescens.[Pubmed:26831947]
Nat Prod Res. 2016 Feb 2:1-6.
Bioassay-guided fractionation of the culture extract of Pestalotiopsis adusta, an endophytic fungus isolated from the medicinal plant Clerodendrum canescens, led to the isolation of one new, (10S)-12,16-epoxy-17(15-->16)-abeo-3,5,8,12,15-abietapentaen-2,7,11,14-tetraone (1), and four known diterpenoids, teuvincenone F (2), Uncinatone (3), coleon U (4), coleon U-12-methyl ether (5). These structures were identified by using spectroscopic methods, including UV, MS, 1D and 2D NMR experiments. This is the first report of these compounds being isolated from a Pestalotiopsis species. The cytotoxic activities of the compounds were evaluated, and compounds 1 and 3 demonstrated cytotoxic activities against the HL-60 tumour cell line (IC50 20 muM).
A new triterpenoid bearing octacosanoate from the stems and roots of Clerodendrum philippinum var. simplex (Verbenaceae).[Pubmed:25801582]
Nat Prod Res. 2015;29(13):1228-34.
A new triterpenoid bearing octacosanoate, named taraxer-3beta-yl octacosanoate (1), together with 13 known compounds (2-14), was isolated from the ethanol extract of the stems and roots of Clerodendrum philippinum var. simplex. The structure of taraxer-3beta-yl octacosanoate (1) was elucidated by extensive spectroscopic analysis. Uncinatone (8) and clerodenone A (10) exhibited inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages with IC(5)(0) values of 12.50 and 3.18 muM, respectively.
Abietane diterpenoids from Clerodendrum bungei.[Pubmed:18348535]
J Nat Prod. 2008 May;71(5):755-9.
Five new naturally occurring abietane diterpenoids (1-5) along with three known diterpenoids (6-8) were isolated from an acetone-soluble extract of the roots of Clerodendrum bungei. The structures of the new compounds were elucidated on the basis of spectroscopic analysis and chemical methods. In addition, all compounds were evaluated for cytotoxic activity against the cultured B16 (murine melanoma), HGC-27 (human gastric), and HEK-293 (human epithelial kidney) cell lines. Uncinatone (7) exhibited moderate cytotoxicity, inhibited cell proliferation, and induced cell-cycle G(2)/M phase arrest.
Anti-complement activity of isolated compounds from the roots of Clerodendrum bungei Steud.[Pubmed:20648692]
Phytother Res. 2010 Nov;24(11):1720-3.
To determine the anti-complement activity of natural diterpenes, chromatographic separation of the acetone-soluble fraction from the roots of Clerodendrum bungei (Verbenaceae) led to the isolation of five diterpenoids. An acetone-soluble extract of the roots of C. bungei exhibited significant anti-complement activity on the classical pathway complement system, which was expressed as total hemolytic activity. Five compounds isolated from the roots of C. bungei, namely 12-O-beta-d-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene (1), 3,12-O-beta-d-diglucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene (2), ajugaside A (3), Uncinatone (4) and 19-hydroxyteuvincenone F (5). Compounds 1, 2, 3, 4 and 5 showed inhibitory activity against complement system with 50% inhibitory concentrations (IC(50)) values of 24 microm, 138 microm, 116 microm, 87 microm and 232 microm. Among the compounds tested, 1 showed the most potent anti-complement activity (IC(50), 24 microm).


