(-)-U-50488 hydrochlorideκ-opioid receptor agonist, selective CAS# 114528-79-9 |
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114528-79-9 | SDF | Download SDF |
| PubChem ID | 9931141 | Appearance | Powder |
| Formula | C19H27Cl3N2O | M.Wt | 405.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
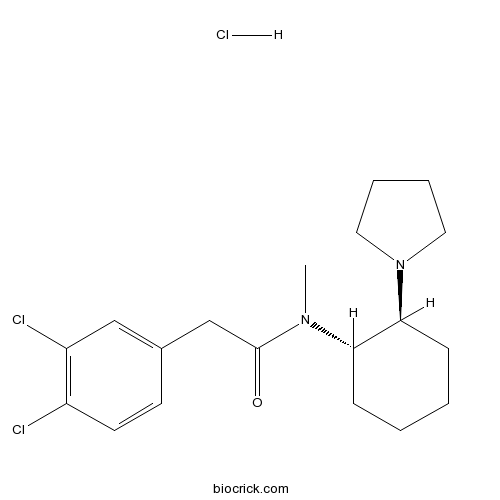
| Chemical Name | 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S,2S)-2-pyrrolidin-1-ylcyclohexyl]acetamide;hydrochloride | ||
| SMILES | CN(C1CCCCC1N2CCCC2)C(=O)CC3=CC(=C(C=C3)Cl)Cl.Cl | ||
| Standard InChIKey | KGMMGVIYOHGOKQ-APTPAJQOSA-N | ||
| Standard InChI | InChI=1S/C19H26Cl2N2O.ClH/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23;/h8-9,12,17-18H,2-7,10-11,13H2,1H3;1H/t17-,18-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | More active enantiomer of (±)-U-50488. (+)-U-50488 hydrochloride also available. |

(-)-U-50488 hydrochloride Dilution Calculator

(-)-U-50488 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4643 mL | 12.3216 mL | 24.6433 mL | 49.2866 mL | 61.6082 mL |
| 5 mM | 0.4929 mL | 2.4643 mL | 4.9287 mL | 9.8573 mL | 12.3216 mL |
| 10 mM | 0.2464 mL | 1.2322 mL | 2.4643 mL | 4.9287 mL | 6.1608 mL |
| 50 mM | 0.0493 mL | 0.2464 mL | 0.4929 mL | 0.9857 mL | 1.2322 mL |
| 100 mM | 0.0246 mL | 0.1232 mL | 0.2464 mL | 0.4929 mL | 0.6161 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(-)-U-50488 hydrochloride is a selective agonist for κ-opioid receptor [1].
The κ-opioid receptor (KOR) is a type of opioid receptor for opioid peptide dynorphin and controls addiction. Also, KOR plays an important role in stress, anxiety, anhedonia, depression and increased drug-seeking behavior.
(-)-U-50488 hydrochloride is a selective KOR agonist [1]. In isolated rat DRG neurons, U-50488 (0.3-40 μM) inhibited voltage-independent Ca2+ channel currents. In HeLa cells that didn’t express KOR, U-50488 (20 μM) blocked Ca2+ channels [2].
In rhesus monkeys, U-50488 exhibited potent antinociceptive activity and produced diuresis [1]. U-50488 enhanced contraction of the rabbit vas deferens induced by electrically with IC50 value of 26.5 nM. In mice, U-50488 impaired motor function with ED50 value of 15.3 mg/kg and reduced spontaneous activity [3]. In adult rats, U-50488 increased the threshold required to maintain self-stimulation responding, a depressive-like effect. While, males were significantly more sensitive than females to the threshold-increasing effects [4].
References:
[1]. Tang AH, Collins RJ. Behavioral effects of a novel kappa opioid analgesic, U-50488, in rats and rhesus monkeys. Psychopharmacology (Berl), 1985, 85(3): 309-314.
[2]. Hassan B, Ruiz-Velasco V. The κ-opioid receptor agonist U-50488 blocks Ca2+ channels in a voltage- and G protein-independent manner in sensory neurons. Reg Anesth Pain Med, 2013, 38(1): 21-27.
[3]. Lu SN, Ma SC, Zhang KG, et al. Comparison of pharmacological profile of selective kappa-opioid agonist K-II and U-50488. Yao Xue Xue Bao, 1991, 26(3): 171-174.
[4]. Russell SE, Rachlin AB, Smith KL, et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry, 2014, 76(3): 213-222.
- Z-Ser-OH
Catalog No.:BCC2743
CAS No.:1145-80-8
- Oroxin B
Catalog No.:BCN1203
CAS No.:114482-86-9
- BNP (1-32), human
Catalog No.:BCC1039
CAS No.:114471-18-0
- Beta-Furoyleupatolide
Catalog No.:BCN6407
CAS No.:114437-24-0
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- PF-8380
Catalog No.:BCC1857
CAS No.:1144035-53-9
- Fmoc-D-Leu-OH
Catalog No.:BCC3511
CAS No.:114360-54-2
- TAK 21d
Catalog No.:BCC5609
CAS No.:1143578-94-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- (+)-U-50488 hydrochloride
Catalog No.:BCC6656
CAS No.:114528-81-3
- Spiramine A
Catalog No.:BCN6023
CAS No.:114531-28-1
- Honyucitrin
Catalog No.:BCN4728
CAS No.:114542-44-8
- Regelidine
Catalog No.:BCN3094
CAS No.:114542-54-0
- Galanin (1-29) (rat, mouse)
Catalog No.:BCC5928
CAS No.:114547-31-8
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
- Ganoderiol F
Catalog No.:BCN6024
CAS No.:114567-47-4
- Coronadiene
Catalog No.:BCN3683
CAS No.:1145689-64-0
- Thevebioside
Catalog No.:BCN6025
CAS No.:114586-47-9
- Soyasaponin Ba
Catalog No.:BCN2854
CAS No.:114590-20-4
- Kisspeptin 234
Catalog No.:BCC6077
CAS No.:1145998-81-7
- Moracin T
Catalog No.:BCN3291
CAS No.:1146113-27-0
Agonist-induced desensitization and down-regulation of the human kappa opioid receptor expressed in Chinese hamster ovary cells.[Pubmed:9535991]
J Pharmacol Exp Ther. 1998 Apr;285(1):28-36.
In this study, we examined whether the human kappa opioid receptor stably expressed in Chinese hamster ovary cells underwent desensitization and down-regulation after prolonged exposure to the agonist (-)U50,488H. Pretreatment with (-)U50,488H led to a reduction in the magnitude of increase in [35S]GTPgammaS binding by the subsequent application of (-)U50,488H. The extent of desensitization was related to duration of exposure and (-)U50,488H concentration. Pretreatment with (-)U50,488H also reduced the potency of (-)U50,488H in inhibiting forskolin-stimulated adenylate cyclase. In membranes of (-)U50,488H-pretreated cells, the affinity of (-)U50,488H was lower than that in the untreated control, and GTPgammaS had no effect on (-)U50,488H affinity, consistent with the notion of uncoupling of the receptor-G protein complex by (-)U50, 488H treatment. Down-regulation of the kappa opioid receptor also occurred on exposure to (-)U50,488H. Higher (-)U50,488H concentrations and/or longer incubation periods were required for down-regulation than for desensitization. The degree of down-regulation depended on the agonist concentration and incubation time. (-)U50,488H-induced desensitization and down-regulation were blocked by naloxone. (+)U50,488H, an inactive stereoisomer, did not cause desensitization or down-regulation. These results indicate that both processes were receptor-mediated. After incubation with (-)U50,488H and removal of (-)U50,488H, both (-)U50,488H-induced [35S]GTPgammaS binding and receptor number returned to the control level, which indicates that both processes were reversible. Thus, desensitization and down-regulation of the kappa opioid receptor occur after agonist exposure and represent two different adaptation mechanisms.
Withdrawal contractures of guinea-pig isolated ileum after acute activation of kappa-opioid receptors.[Pubmed:8388301]
Br J Pharmacol. 1993 May;109(1):48-52.
1. The present study was undertaken to investigate firstly whether a brief exposure for 5 min of guinea-pig isolated ileum to the kappa-opioid agonist, U-50,488H produced a withdrawal contracture on addition of naloxone and secondly to ascertain whether the response was due to the activation of kappa-opioid receptors. 2. Naloxone (10(-6) M) did not elicit a response in preparations exposed to U-50,488H (5 x 10(-7) M-2 x 10(-6) M). However, after exposure to U-50,488H (5 x 10(-7) M), naloxone (10(-6) M) produced a strong contracture if the agonist was washed out 1 min before the addition of the antagonist. 3. The addition of naloxone (10(-6) M) to the ileum preparation exposed to U-50,488H (10(-7) M or lower) caused a response of similar intensity irrespective of whether the agonist had been washed out. 4. The selective kappa-opioid antagonist, nor-binaltorphimine (2.7 x 10(-9) M and 2.7 x 10(-7) M), injected before the opioid agonists, prevented the naloxone-induced contracture after exposure to U-50,488H (8 x 10(-8) M) but did not affect the contracture after exposure to morphine (5 x 10(-7) M). 5. Nor-binaltorphimine (2.7 x 10(-9) M) caused a contraction of the ileum preparation when injected 5 min after exposure to U-50,488H (8 x 10(-8) M) but not after morphine (5 x 10(-7) M). 6. The alpha 2-adrenoceptor agonist, clonidine (3 x 10-8 M) and the calcium channel blocker, nifedipine(3 x 10-8 M), injected 1 min before naloxone, blocked the ileum contraction to naloxone after exposure to U-50,488H (8 x 10-8 M). The results demonstrate that the stimulation of Kappa-opioid receptors can induce a similar dependence in guinea-pig ileum to that produced by activation of micro receptors.
Effect of chronic administration of U-50,488H, a kappa-opioid receptor agonist, on central dopamine D2 receptors of the rat.[Pubmed:8390939]
Eur J Pharmacol. 1993 Apr 22;235(1):23-30.
The effect of U-50,488H, a kappa-opiate agonist, induced tolerance and abstinence on the characteristics of dopamine D2 receptors of brain regions and spinal cord was determined in the rat. Male Sprague-Dawley rats were injected with U-50,488H (25 mg/kg i.p) or its vehicle twice a day for 4 days. This procedure resulted in the development of tolerance to the analgesic activity of U-50,488H. The binding characteristics (Bmax and Kd values) of [3H]spiroperidol to dopamine D2 receptors were determined in discrete brain regions (hypothalamus, hippocampus, cortex, pons and medulla, midbrain, corpus striatum and amygdala) and spinal cord of U-50,488H-tolerant and -abstinent rats. Rats labeled as tolerant to U-50,488H were injected with U-50,488H on day 5 and killed 1 h later, whereas those labeled abstinent were killed on day 5 without the injection of the drug. Vehicle-injected rats served as controls. [3H]Spiroperidol bound to brain regions and spinal cord membranes at a single high affinity site. The Bmax and Kd values of [3H]spiroperidol in brain regions and spinal cord of U-50,488H-tolerant and abstinent rats did not differ from their respective vehicle-injected controls. The behavioral responses (total distance travelled, horizontal activity, movement time, total number of movements, number of stereotypic movements, stereotypic time and rest time) to different doses of a selective dopamine D2 receptor agonist, 2-bromo-alpha-ergocryptine (bromocriptine) were also determined in rats treated chronically with U-50,488H.(ABSTRACT TRUNCATED AT 250 WORDS)
Synthesis and absolute configuration of optically pure enantiomers of a kappa-opioid receptor selective agonist.[Pubmed:2822490]
FEBS Lett. 1987 Nov 2;223(2):335-9.
The enantiomers of U50,488, ligands highly selective for kappa-opioid receptors, have been prepared by a refined procedure and their optical purity demonstrated. The absolute configuration of (+)-trans-2-pyrrolidinyl-N-methylcyclohexylamine, a chemically versatile intermediate for synthesis of analogs of kappa-opioid receptor ligands with defined chirality, has been determined to be 1S,2S by X-ray crystallographic analysis. This intermediate has been used to synthesize the optically pure U50,488 enantiomers with known absolute configuration.


