TriamcinoloneCAS# 124-94-7 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124-94-7 | SDF | Download SDF |
| PubChem ID | 31307 | Appearance | Powder |
| Formula | C21H27FO6 | M.Wt | 394.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (253.53 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
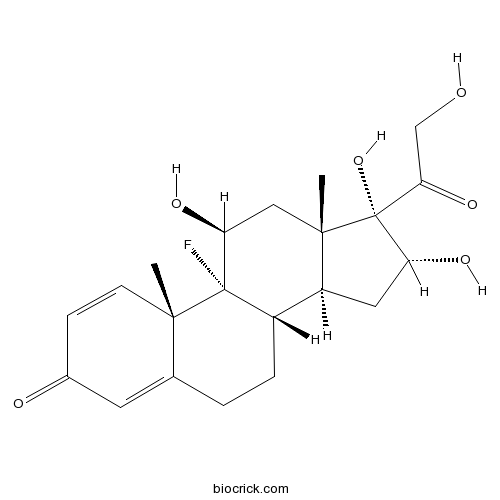
| Chemical Name | (8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12CC(C3(C(C1CC(C2(C(=O)CO)O)O)CCC4=CC(=O)C=CC43C)F)O | ||
| Standard InChIKey | GFNANZIMVAIWHM-OBYCQNJPSA-N | ||
| Standard InChI | InChI=1S/C21H27FO6/c1-18-6-5-12(24)7-11(18)3-4-13-14-8-15(25)21(28,17(27)10-23)19(14,2)9-16(26)20(13,18)22/h5-7,13-16,23,25-26,28H,3-4,8-10H2,1-2H3/t13-,14-,15+,16-,18-,19-,20-,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triamcinolone is a long-acting synthetic corticosteroid.
Target: Glucocorticoid Receptor
Dimethyl fumarate is an anti-inflammatory. It is indicated for multiple sclerosis patients with relapsing forms and is also being investigated for the treatment of psoriasis. The mechanism of action of dimethyl fumarate in multiple sclerosis is not well understood. It is thought to involve dimethyl fumarate degradation to its active metabolite monomethyl fumarate (MMF) then MMF up-regulates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway that is activated in response to oxidative stress [1].
The mean duration of follow-up was 40 months. The rate of decline in the FEV1 after bronchodilator use was similar in the 559 participants in the triamcinolone group and the 557 participants in the placebo group (44.2+/-2.9 vs. 47.0+/-3.0 ml per year, P= 0.50). Members of the triamcinolone group had fewer respiratory symptoms during the course of the study (21.1 per 100 person-years vs. 28.2 per 100 person-years, P=0.005) and had fewer visits to a physician because of a respiratory illness (1.2 per 100 person-years vs. 2.1 per 100 person-years, P=0.03). Those taking triamcinolone also had lower airway reactivity in response to methacholine challenge at 9 months and 33 months (P=0.02 for both comparisons) [2]. References: | |||||

Triamcinolone Dilution Calculator

Triamcinolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5353 mL | 12.6765 mL | 25.353 mL | 50.7061 mL | 63.3826 mL |
| 5 mM | 0.5071 mL | 2.5353 mL | 5.0706 mL | 10.1412 mL | 12.6765 mL |
| 10 mM | 0.2535 mL | 1.2677 mL | 2.5353 mL | 5.0706 mL | 6.3383 mL |
| 50 mM | 0.0507 mL | 0.2535 mL | 0.5071 mL | 1.0141 mL | 1.2677 mL |
| 100 mM | 0.0254 mL | 0.1268 mL | 0.2535 mL | 0.5071 mL | 0.6338 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Triamcinolone
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- Kazinol U
Catalog No.:BCN4720
CAS No.:1238116-48-7
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
- Kobophenol A
Catalog No.:BCN3444
CAS No.:124027-58-3
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
- 1-Caffeoylquinic acid
Catalog No.:BCN5911
CAS No.:1241-87-8
- 2-Hydroxytetracosanoic acid ethyl ester
Catalog No.:BCN1599
CAS No.:124111-47-3
- Scutebarbatine O
Catalog No.:BCN8377
CAS No.:960302-88-9
- Alcesefoliside
Catalog No.:BCN2933
CAS No.:124151-38-8
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
Intravitreal bevacizumab alone or combined with 1 mg triamcinolone in diabetic macular edema: a randomized clinical trial.[Pubmed:28349504]
Int Ophthalmol. 2018 Apr;38(2):585-598.
PURPOSE: To compare the results of intravitreal bevacizumab (IVB) injection alone or in combination with intravitreal 1 mg Triamcinolone acetonide (IVT) in center-involved diabetic macular edema. METHODS: In this randomized clinical trial study, ninety-two eyes of 46 patients with bilateral center-involved diabetic macular edema and no previous treatment were included in the study. One eye of each patient was randomly assigned to 1.25 mg of IVB injection or combination of 1.25 IVB and 1 mg IVT. Evaluation of best-corrected visual acuity (BCVA), central macular thickness (CMT), intraocular pressure (IOP) and grading of lens opacity was conducted at baseline, and weeks 2, 4, 6, 8, 12 and 24 after treatment. Retreatment was performed at a 6-week interval whenever indicated based on CMT. RESULTS: Between the groups, BCVA changes were not statistically different until 24-week follow-up (P > 0.05), but at 24 weeks after treatment, BCVA improvement was significantly better in IVB group (P = 0.049). Significant CMT reduction was observed in each group along the follow-up period (P = 0.001). The mean CMT reduction was more significant in combination (IVB + IVT) group at 2 weeks of follow-up (P < 0.001), but CMT changes were not significant between the groups at weeks 12th and 24th after injection. Overall, retreatment was applied for 59 eyes up to 24 weeks (33 in the IVB group, 26 in the IVB + IVT group). Among patients with 2 or more injections, number of injections was significantly lower in IVB + IVT group (P = 0.043). Three eyes within IVB + IVT group developed IOP rise beyond 21 mmHg, which were controlled with topical anti-glaucoma medications within 1 week. Changes in lens opacity were not significant between two groups. CONCLUSION: Eyes treated with IVB plus 1 mg IVT injections had more significant reduction in CMT in early post-injection, but this effect was transient. Although after 24 weeks visual acuity improvement was better in IVB group, combination therapy may decrease the number of injections. Combining 1 mg of intravitreal Triamcinolone with bevacizumab was not accompanied with significant side effects.
A Prospective Randomized Trial of the Efficacy of Fibrin Glue, Triamcinolone Acetonide, and Quilting Sutures in Seroma Prevention after Latissimus Dorsi Breast Reconstruction.[Pubmed:28350654]
Plast Reconstr Surg. 2017 Apr;139(4):854e-863e.
BACKGROUND: Donor-site seroma is the most common complication following latissimus dorsi flap breast reconstruction. Various agents and techniques have attempted to minimize seroma formation. The purpose of this study was to compare the efficacy of different products and quilting sutures at seroma prevention. METHODS: This is a single-center, double-blinded, randomized, controlled trial of a consecutive series of breast cancer patients (n = 96) undergoing latissimus dorsi flap reconstruction performed by a single surgeon. Patients were randomized to receive (1) fibrin glue (Tisseel) (n = 23), (2) Triamcinolone acetonide (n = 26), or (3) normal saline (control) (n = 27) sprayed into the donor site. The fourth arm included donor-site quilting sutures (n = 20). Outcomes included seroma, drain output, and days to last drain removal. Drain removal was standardized at less than 30 cc/day. RESULTS: All groups were matched evenly without differences in risk, procedures, or complications. The overall seroma rate was 31.3 percent (n = 30). The quilting group had significantly less drainage for weeks 1 (p = 0.006) and 2 (p = 0.050) postoperatively. Quilting statistically reduced the incidence of seromas to 5.0 percent (n = 1; p = 0.038) compared with other groups (control, 34.5 percent; fibrin, 27.6 percent; and Triamcinolone, 37.6 percent). Drains were removed 10 days earlier with quilting (control, 35.5 days; fibrin, 39.5 days; Triamcinolone, 37.4 days; and quilting, 25.8 days; p = 0.001). The incidence of all other complications was similar between groups. CONCLUSION: The use of quilting donor sites significantly decreases the incidence of donor-site seromas and leads to earlier drain removal following latissimus dorsi flap reconstruction and maintains a low complication profile. CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, II.
Intra-articular injection with triamcinolone hexacetonide in patients with rheumatoid arthritis: prospective assessment of goniometry and joint inflammation parameters.[Pubmed:28343615]
Rev Bras Reumatol Engl Ed. 2017 Mar - Apr;57(2):115-121.
OBJECTIVES: To evaluate local joint variables after intra-articular injection with Triamcinolone hexacetonide in rheumatoid arthritis patients. METHODS: We blindly and prospectively (baseline, 1, 4, 12 and 24 weeks) evaluated metacarpophalangeal, wrist, elbow, shoulder, knee and ankle joints after Triamcinolone hexacetonide intra-articular injection by the following outcome measures: visual analogue scale 0-10cm (VAS) for rest pain (VASR); VAS for movement pain (VASM); VAS for joint swelling (VASSw); flexion (FlexG) and extension (ExtG). RESULTS: 289 patients (635 joints) were studied. VASSw (p<0.001) and VASR (0.001
Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres.[Pubmed:28342981]
J Control Release. 2017 Jun 28;256:19-25.
Little is known about the underlying effects controlling in vitro-in vivo correlations (IVIVCs) for biodegradable controlled release microspheres. Most reports of IVIVCs that exist are empirical in nature, typically based on a mathematical relationship between in vitro and in vivo drug release, with the latter often estimated by deconvolution of pharmacokinetic data. In order to improve the ability of in vitro release tests to predict microsphere behavior in vivo and develop more meaningful IVIVCs, the in vivo release mechanisms need to be characterized. Here, two poly(lactic-co-glycolic acid) (PLGA) microsphere formulations encapsulating the model steroid Triamcinolone acetonide (Tr-A) were implanted subcutaneously in rats by using a validated cage model, allowing for free fluid and cellular exchange and microsphere retrieval during release. Release kinetics, as well as mechanistic indicators of release such as hydrolysis and mass loss, was measured by direct analysis of the recovered microspheres. Release of Tr-A from both formulations was greatly accelerated in vivo compared to in vitro using agitated phosphate buffered saline +0.02% Tween 80 pH7.4, including rate of PLGA hydrolysis, mass loss and water uptake. Both microsphere formulations exhibited erosion-controlled release in vitro, indicated by similar polymer mass loss kinetics, but only one of the formulations (low molecular weight, free acid terminated) exhibited the same mechanism in vivo. The in vivo release of Tr-A from microspheres made of a higher molecular weight, ester end-capped PLGA displayed an osmotically induced/pore diffusion mechanism based on confocal micrographs of percolating pores in the polymer, not previously observed in vitro. This research indicates the need to fully understand the in vivo environment and how it causes drug release from biodegradable microspheres. This understanding can then be applied to develop in vitro release tests which better mimic this environment and cause drug release by the relevant mechanistic processes, ultimately leading to the development of mechanism based IVIVCs.


