TopotecanTopoisomerase 1 inhibitor CAS# 123948-87-8 |
Quality Control & MSDS
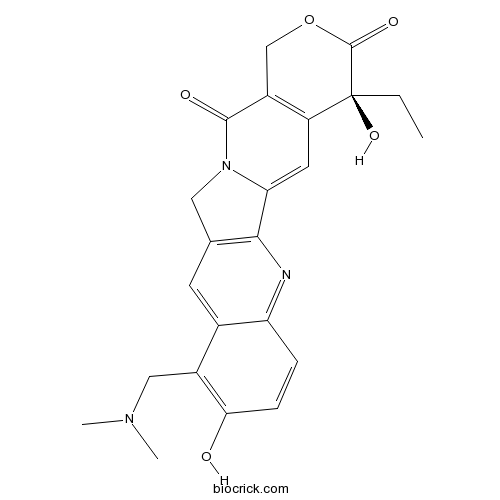
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123948-87-8 | SDF | Download SDF |
| PubChem ID | 60700 | Appearance | Powder |
| Formula | C23H23N3O5 | M.Wt | 421.45 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | SKF104864 | ||
| Solubility | >21.1mg/mL in DMSO | ||
| SMILES | CCC1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=C(C5=C4)CN(C)C)O)O | ||
| Standard InChIKey | UCFGDBYHRUNTLO-QHCPKHFHSA-N | ||
| Standard InChI | InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TLyp-1-functionalized topotecan-loaded niosomes could significantly improve anti-glioma treatment. Preparation of topotecan into a temperature-sensitive phase-change hydrogel achieves a long-term sustained antitumor effect on Rb cells, and may be a useful strategy for the treatment of intraocular Rb. |
| In vitro | A temperature-sensitive phase-change hydrogel of topotecan achieves a long-term sustained antitumor effect on retinoblastoma cells.[Pubmed: 31534347 ]Onco Targets Ther. 2019 Jul 30;12:6069-6082.Retinoblastoma (Rb) is one of the most common malignancies among children. Following early diagnosis and prompt treatment, the clinical outcome or prognosis of Rb is promising. However, the prognosis or survival rates of patients with late-stage Rb remain poor. Current therapeutic strategies for advanced Rb mainly involve the use of advanced chemotherapeutic options. However, the efficacy of these strategies is not satisfactory. Therefore, the development of novel strategies to achieve a more effective antitumor effect on late-stage Rb is of crucial importance.
|
| Structure Identification | Int J Mol Sci. 2019 Sep 22;20(19). pii: E4696.Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery.[Pubmed: 31546717 ]Niosomes are non-ionic surfactant-based vesicles with high promise for drug delivery applications. They can be rapidly prepared via microfluidics, allowing their reproducible production without the need of a subsequent size reduction step, by controlled mixing of two miscible phases of an organic (lipids dissolved in alcohol) and an aqueous solution in a microchannel. The control of niosome properties and the implementation of more complex functions, however, thus far are largely unknown for this method.
|

Topotecan Dilution Calculator

Topotecan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3728 mL | 11.8638 mL | 23.7276 mL | 47.4552 mL | 59.319 mL |
| 5 mM | 0.4746 mL | 2.3728 mL | 4.7455 mL | 9.491 mL | 11.8638 mL |
| 10 mM | 0.2373 mL | 1.1864 mL | 2.3728 mL | 4.7455 mL | 5.9319 mL |
| 50 mM | 0.0475 mL | 0.2373 mL | 0.4746 mL | 0.9491 mL | 1.1864 mL |
| 100 mM | 0.0237 mL | 0.1186 mL | 0.2373 mL | 0.4746 mL | 0.5932 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Topotecan (SKF104864) is an inhibitor of topoisomerase 1 and semisynthetic analogue of camptothecin [1].
Topotecan (SKF104864) has been reported to have a potent antitumor activity against tumors in murine models. In addition, Topotecan has also shown the potent effect against intravenously implanted P388 leukemia and both intravenously and subcutaneously implanted Lewis lung carcinoma. Topotecan has noted the activity against subcutaneously implanted solid tumors including chemorefractory tumors and human colon carcinoma xenograft HT-29. Topotecan has been found to induce regressions in the lung tumor model (Lewis lung carcinoma and B16 melanoma), compared to camptothecin and 9-amino-camptothecin. In the preclinical toxicology studies, Topotecan has been revealed to have a concentration-dependent, reversible and limited toxoicity to rapidly proliferation tissues such as bone marrow and gastro-intestinal epithelium [1].
References:
[1] Creemers GJ1, Lund B, Verweij J. Topoisomerase I inhibitors: topotecan and irenotecan. Cancer Treat Rev. 1994 Jan;20(1):73-96.
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- Kazinol U
Catalog No.:BCN4720
CAS No.:1238116-48-7
- Hopeachinol B
Catalog No.:BCN3445
CAS No.:1238083-45-8
- Hydroxyevodiamine
Catalog No.:BCN2491
CAS No.:1238-43-3
- QNZ 46
Catalog No.:BCC6292
CAS No.:1237744-13-6
- ML 786 dihydrochloride
Catalog No.:BCC7997
CAS No.:1237536-18-3
- Escin IA
Catalog No.:BCN3862
CAS No.:123748-68-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
- Kobophenol A
Catalog No.:BCN3444
CAS No.:124027-58-3
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
Topotecan-Vincristine-Doxorubicin in Stage 4 High-Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC: A SIOPEN Study.[Pubmed:28324923]
Cancer Res Treat. 2018 Jan;50(1):148-155.
PURPOSE: Metastatic response to induction therapy for high-risk neuroblastoma is a prognostic factor. In the International Society of Paediatric Oncology Europe Neuroblastoma (SIOPEN) HR-NBL-1 protocol, only patients with metastatic complete response (CR) or partial response (PR) with Topotecan-vincristine-doxorubicin (TVD) was evaluated in patients failing to achieve these criteria, with the aim of improving the metastatic response rate. MATERIALS AND METHODS: Patients with metastatic high-risk neuroblastoma who had not achieved the SIOPEN criteria for HDT after induction received two courses of Topotecan 1.5 mg/m(2)/day for 5 days, followed by a 48-hour infusion of vincristine, 2 mg/m(2), and doxorubicin, 45 mg/m(2). RESULTS: Sixty-three patients were eligible and evaluable. Following two courses of TVD, four (6.4%) patients had an overall CR, while 28 (44.4%) had a PR with a combined response rate of 50.8% (95% confidence interval [CI], 37.9 to 63.6). Of these, 23 patients achieved a metastatic CR or a PR with
Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers.[Pubmed:28283779]
Invest New Drugs. 2017 Oct;35(5):627-633.
Background This multicenter, open-label, phase Ib study was designed to assess the safety, pharmacokinetics and preliminary efficacy of ME-344, a mitochondrial inhibitor, administered in combination with the topoisomerase I inhibitor, Topotecan, in patients with previously treated, locally advanced or metastatic small cell lung (SCLC), ovarian and cervical cancers. Patients and methods In Part 1, patients received ME-344 10 mg/kg intravenously weekly on days 1, 8, 15 and 22 in combination with Topotecan 4 mg/m(2) on days 1, 8, and 15 of a 28 day cycle. Cycles were repeated until disease progression or unacceptable toxicity. Patients were evaluated for dose-limiting toxicity (DLT) in cycle 1 and ME-344 pharmacokinetic samples were obtained. In Part 2, patients with locally advanced or metastatic SCLC and ovarian cancer were enrolled in expansion cohorts treated at the recommended phase II dose (RP2D) determined in Part 1. Results Fourteen patients were enrolled in Part 1 and no DLTs were observed. The RP2D of ME-344 in combination with Topotecan was established as 10 mg/kg. In Part 2, 32 patients were enrolled. The most common treatment-emergent all-grade and grade 3/4 toxicities included fatigue (65.2%, 6.5%), neutropenia (56.5%, 43.5%) and thrombocytopenia (50%, 23.9%). One patient with recurrent ovarian cancer experienced a partial response by RECIST 1.1 and 21 patients achieved stable disease as best response. Conclusions The combination of ME-344 10 mg/kg weekly and Topotecan 4 mg/m(2) was tolerable, however, the degree of anti-cancer activity does not support further investigation of the combination in unselected patients with SCLC, ovarian and cervical cancers.
New and Old Genes Associated with Topotecan Resistance Development in Ovarian Cancer Cell Lines.[Pubmed:28373423]
Anticancer Res. 2017 Apr;37(4):1625-1636.
BACKGROUND: Low effectiveness of chemotherapy in ovarian cancer results from development of drug resistance. Topotecan is a drug used as second-line chemotherapy for this cancer type. We analyzed development of Topotecan resistance in ovarian cancer cell lines. MATERIALS AND METHODS: A chemosensitivity assay, MTT test, was performed to assess drug resistance. Quantitative polymerase chain reaction (Q-PCR) assays were performed to determine ABCB1, ABCG2, ALDH1A1, IFIH1, SAMD4 and EPHA3 gene expression. RESULTS: We observed dose-dependent responses to Topotecan. In all Topotecan-resistant cell lines an overexpression of ABCG2, IFIH1 and SAMD4 genes was observed. Expression of ABCB1 gene was observed in one cell line. Expression of ALDH1A1 was up-regulated in A2780 and down-regulated in SKOV-3-resistant cell lines. Short-time exposure led to similar patterns of gene expression for the investigated genes. CONCLUSION: Expression of ABCG2 and ABCB1 genes plays the most important role in Topotecan resistance. The role of other investigated genes seems to be complementary.
Phase II randomized study of PM01183 versus topotecan in patients with platinum-resistant/refractory advanced ovarian cancer.[Pubmed:28368437]
Ann Oncol. 2017 Jun 1;28(6):1280-1287.
Background: PM01183 is a new compound that blocks active transcription, produces DNA breaks and apoptosis, and affects the inflammatory microenvironment. PM01183 showed strong antitumor activity in preclinical models of cisplatin-resistant epithelial ovarian cancer. Patients and methods: Patients with platinum-resistant/refractory ovarian cancer were included in a two-stage, controlled, randomized (in a second stage), multicenter, phase II study. Primary endpoint was overall response rate (ORR) by RECIST and/or GCIG criteria. The exploratory first stage (n = 22) confirmed the activity of PM01183 as a single agent at 7.0 mg flat dose every 3 weeks (q3wk). The second stage (n = 59) was randomized and controlled with Topotecan on days 1-5 q3wk or weekly (every 4 weeks, q4wk). Results: ORR was 23% (95% CI, 13%-37%) for 52 PM01183-treated patients. Median duration of response was 4.6 months (95% CI, 2.5-6.9 months), and 23% (95% CI, 0%-51%) of responses lasted 6 months or more. Ten of the 12 confirmed responses were reported for 33 patients with platinum-resistant disease [ORR = 30% (95% CI, 16%-49%)]; for the 29 patients treated with Topotecan in the second stage, no responses were found. Median PFS for all PM01183-treated patients was 4.0 months (95% CI, 2.7-5.6 months), and 5.0 months (95% CI, 2.7-6.9 months) for patients with platinum-resistant disease. Grade 3/4 neutropenia in 85% of patients; febrile neutropenia in 21% and fatigue (grade 3 in 35%) were the principal safety findings for PM01183. Conclusion: PM01183 is an active drug in platinum-resistant/refractory ovarian cancer and warrants further development. The highest activity was observed in platinum-resistant disease. Its safety profile indicates the dose should be adjusted to body surface area (mg/m2). Trial code: EudraCT 2011-002172-16.


