TolvaptanAVP V2-receptor antagonist CAS# 150683-30-0 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150683-30-0 | SDF | Download SDF |
| PubChem ID | 443894 | Appearance | Powder |
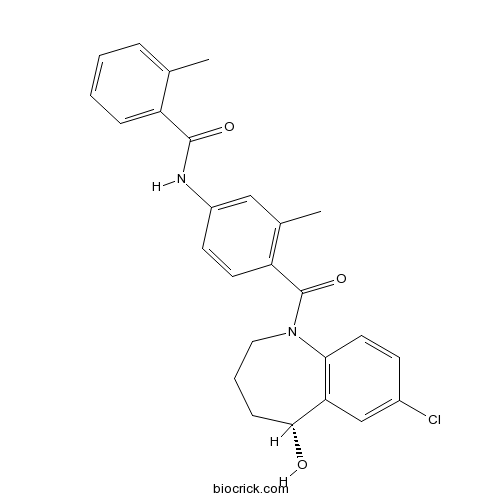
| Formula | C26H25ClN2O3 | M.Wt | 448.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | OPC-41061 | ||
| Solubility | DMSO : ≥ 100 mg/mL (222.75 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[4-[(5R)-7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl]-3-methylphenyl]-2-methylbenzamide | ||
| SMILES | CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCCC(C4=C3C=CC(=C4)Cl)O)C | ||
| Standard InChIKey | GYHCTFXIZSNGJT-XMMPIXPASA-N | ||
| Standard InChI | InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31)/t24-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective competitive vasopressin V2 receptor antagonist (Ki values are 0.06 and 12.3 nM for V2 and V1a receptors respectively). Decreases urine osmolality and increases serum sodium concentrations. Delays the onset of end-stage renal disease in a mouse model of polycystic kidney disease. Exhibits myocardial and renal protective effects in hypertensive heart failure rats. Orally active. |

Tolvaptan Dilution Calculator

Tolvaptan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2275 mL | 11.1373 mL | 22.2747 mL | 44.5494 mL | 55.6867 mL |
| 5 mM | 0.4455 mL | 2.2275 mL | 4.4549 mL | 8.9099 mL | 11.1373 mL |
| 10 mM | 0.2227 mL | 1.1137 mL | 2.2275 mL | 4.4549 mL | 5.5687 mL |
| 50 mM | 0.0445 mL | 0.2227 mL | 0.4455 mL | 0.891 mL | 1.1137 mL |
| 100 mM | 0.0223 mL | 0.1114 mL | 0.2227 mL | 0.4455 mL | 0.5569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolvaptan is a selective and oral active antagonist of arginine vasopressin (AVP) V2-receptor with Ki value of 0.43nM [1].
Tolvaptan is a nonpeptide AVP V2-receptor antagonist. It prevents AVP from binding to V2-receptor. In the in vitro binding assay using HeLa cells expressing human AVP receptor subtypes, tolvaptan shows inhibitory activity against V2 and V1a receptors with Ki values of 0.43nM and 12.3nM, respectively. The V1b receptor and 30 other receptors or ion channels are not sensitive to tolvaptan, indicating that tolvaptan is selective to V2-receptor. Tolvaptan also inhibits the production of cAMP induced by AVP with IC50 value of 8nM [1].
In animal models, the tolvaptan induced aquaresis results in increased urine volume and serum sodium. In rat models of acute and chronic hyponatremia, administration of tolvaptan increases plasma sodium levels and decreases the mortality [1].
References:
[1] Miyazaki T, Fujiki H, Yamamura Y, et al. Tolvaptan, an Orally Active Vasopressin V2-Receptor Antagonist-Pharmacology and Clinical Trials. Cardiovascular drug reviews, 2007, 25(1): 1-13.
- Fmoc-Lys(Dde)-OH
Catalog No.:BCC3518
CAS No.:150629-67-7
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- L-NIL hydrochloride
Catalog No.:BCC5706
CAS No.:150403-89-7
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- SR 49059
Catalog No.:BCC7324
CAS No.:150375-75-0
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Prasugrel
Catalog No.:BCC1089
CAS No.:150322-43-3
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
- Oxybutynin chloride
Catalog No.:BCC4149
CAS No.:1508-65-2
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Retigabine
Catalog No.:BCC6427
CAS No.:150812-12-7
- Retigabine dihydrochloride
Catalog No.:BCC1890
CAS No.:150812-13-8
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
- Nitenpyram
Catalog No.:BCC5559
CAS No.:150824-47-8
- EGF816
Catalog No.:BCC6428
CAS No.:1508250-71-2
- Boc-Orn(Fmoc)-OH
Catalog No.:BCC3429
CAS No.:150828-96-9
Hyponatraemia and congestive heart failure refractory to diuretic treatment. Utility of tolvaptan.[Pubmed:28372784]
Rev Clin Esp. 2017 Oct;217(7):398-404.
Heart failure (HF) is currently one of the most significant healthcare problems in Spain and has a continuously increasing prevalence. Advances in our understanding of the various biological responses that promote cardiac remodelling and pulmonary venous congestion constitute the basis of current treatment. This article, prepared by members of the HF groups of the Spanish Society of Cardiology and the Spanish Society of Internal Medicine, discusses the current therapeutic strategies for patients with congestion refractory to diuretic treatment. The article includes our clinical experience with the use of Tolvaptan as an additional treatment for congestion associated with hyponatraemia. To this end, we propose an algorithm for the use of Tolvaptan in patients with congestive HF, natraemia <130mEq/l and poor response to conventional diuretic treatment.
Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial.[Pubmed:28379536]
Nephrol Dial Transplant. 2018 Mar 1;33(3):477-489.
Background: In TEMPO 3:4, the vasopressin V2 receptor antagonist Tolvaptan slowed total kidney volume (TKV) growth and estimated glomerular filtration rate (eGFR) decline relative to placebo. Methods: TEMPO 4:4 was designed to provide an additional 2 years of data on the long-term safety and efficacy of Tolvaptan in subjects completing TEMPO 3:4. The objective was to assess the disease-modifying effects of Tolvaptan on TKV and eGFR end-points including change from baseline over the combined duration of TEMPO 3:4 and TEMPO 4:4, and non-inferiority of slopes during TEMPO 4:4. Results: Of the 1445 subjects randomized to TEMPO 3:4, 871 (60.3%) enrolled in TEMPO 4:4. Percent changes in TKV from TEMPO 3:4 baseline to TEMPO 4:4 Month 24 were 29.9% and 31.6% (prior Tolvaptan versus prior placebo, P = 0.38). Adjusting for baseline covariates improved the TKV treatment difference at Month 24 in TEMPO 4:4 from -1.70% to - 4.15% between the groups (P = 0.04). Slopes of TKV growth during TEMPO 4:4 were higher in early- versus delayed-treatment groups (6.16% versus 4.96% per year, P = 0.05). Analysis of secondary eGFR endpoints demonstrated a persistent effect on eGFR (3.15 mL/min/1.73 m2, P < 0.001), and non-inferiority in eGFR slopes. The safety profile on exposure to Tolvaptan in TEMPO 4:4 was similar to that in TEMPO 3:4. Conclusions: The results of TEMPO 4:4 support a sustained disease-modifying effect of Tolvaptan on eGFR. The lack of a sustained treatment difference on TKV may be accounted for by limitations of the trial design, including loss of randomization and baseline imbalances ensuing TEMPO 3:4. The safety profile was similar to that observed in TEMPO 3:4.
Prediction of diuretic response to tolvaptan by a simple, readily available spot urine Na/K ratio.[Pubmed:28362879]
PLoS One. 2017 Mar 31;12(3):e0174649.
BACKGROUND: Tolvaptan is vasopressin type 2 receptor antagonist that inhibits water reabsorption. It is used in combination with standard diuretics to treat ascites unresponsive to standard diuretic therapy or hyponatremia because of liver cirrhosis. This study evaluated the effectiveness and safety of Tolvaptan in clinical practice and aimed to determine the factors related to its effectiveness. METHODS: Tolvaptan was administered to 88 consecutive cirrhotic patients with ascites unresponsive to standard diuretic therapy. An effective treatment response was a >/=2% reduction in body weight on day 7. The association of patient pretreatment characteristics with therapeutic effects was analyzed. RESULTS: Mean weight reduction on day 7 of Tolvaptan therapy was -2.9% +/- 3.2%, and treatment was effective in 52% of patients. Multivariate analysis revealed that spot urine Na/K ratio >/=2.5 at baseline was the only factor independently related to therapeutic effect, with an odds ratio of 7.85 (95% confidence interval 2.64-23.40, p = 0.0002). Weight reduction percentage on day 7 was -4.0% +/- 2.8% in patients with spot urine Na/K >/=2.5, which was significantly greater than the 0.7% +/- 2.7% loss in those with urine Na/K < 2.5 (p < 0.05). A spot urine Na/K ratio >/=2.5 had a sensitivity of 85% and specificity of 60% for predicting effective treatment. No adverse events of treatment led to treatment discontinuation. CONCLUSIONS: Baseline spot urine Na/K was predictive of an effective response to Tolvaptan therapy. It is simple to perform and readily available and might serve as an indicator of optimal timing of Tolvaptan administration in patients with inadequate response to conventional Na diuretic therapy.
Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury.[Pubmed:24570071]
J Pharmacol Exp Ther. 2014 May;349(2):258-67.
Tolvaptan, a selective vasopressin V2 receptor antagonist, slows the increase in total kidney volume and the decline in kidney function in patients with the results of the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Outcome (TEMPO) 3:4 trial. However, it was unclear which dose of Tolvaptan was optimal or whether Tolvaptan was able to delay progression to end-stage renal disease (ESRD). Here we examined the relationship with aquaresis and the inhibitory effect on cyst development in short-term treatment and mortality as an index of ESRD in long-term treatment with Tolvaptan using DBA/2FG-pcy mice, an animal model of nephronophthisis. With short-term treatment from 5 to 15 weeks of age, Tolvaptan (0.01-0.3% via diet) dose-dependently enhanced aquaresis, prevented increases in kidney weight and cyst volume, and was associated with significant reductions in kidney cAMP levels and extracellular signal-regulated kinase activity. Maximal effects of Tolvaptan on aquaresis and the prevention of development of polycystic kidney disease (PKD) were obtained at 0.1%. Interestingly, Tolvaptan also dose-dependently reduced urinary neutrophil gelatinase-associated lipocalin levels in correlation with the kidney volume. With long-term treatment from 5 to 29 weeks of age, Tolvaptan significantly attenuated the increase in kidney volume by up to 50% and reduced urinary albumin excretion. Furthermore, Tolvaptan significantly reduced the mortality rate to 20%, compared with 60% in the control group. These data indicate that Tolvaptan may delay the onset of ESRD in PKD by suppressing the increases in kidney volume and renal injury, providing a promising treatment for PKD.
Chronic administration of oral vasopressin type 2 receptor antagonist tolvaptan exerts both myocardial and renal protective effects in rats with hypertensive heart failure.[Pubmed:22628529]
Circ Heart Fail. 2012 Jul 1;5(4):484-92.
BACKGROUND: Although recent clinical trials have demonstrated the efficacy of the oral vasopressin (AVP) type 2 receptor (V2R) antagonist Tolvaptan, its long-term effects on the myocardium and kidney in heart failure (HF) are not clear. We examined the chronic effects of Tolvaptan administration on both the myocardium and kidney in a rat hypertensive HF model. METHODS AND RESULTS: Not only circulating AVP level but also myocardial AVP and V1a receptor (V1aR) expressions, renal V1aR, and V2R expressions were significantly upregulated during the transition to HF. The animals were chronically treated with low-dose or high-dose (HD) Tolvaptan or vehicle from the left ventricular (LV) hypertrophic stage. Chronic Tolvaptan treatment persistently increased urine volume but did not affect blood pressure. In the HD group, the animal survival significantly improved (log-rank test, P<0.01). At the HF stage, the progression of LV dysfunction was prevented and lung congestion was suppressed. Activation of atrial natriuretic peptide, endothelin-1, AVP, and V1aR mRNA levels were significantly suppressed in the LV myocardium. Meanwhile, renal histopathologic damage was ameliorated and renal function was improved in the HD group at the HF stage. Concomitantly, not only activation of aquaporin-2 but also those of V2R, V1aR, renin, and endothelin-1 in the kidney were significantly suppressed (all P<0.05). CONCLUSIONS: These results indicate that chronic Tolvaptan treatment has beneficial effects by preventing not only the progression of LV dysfunction but also that of renal injury in hypertensive rats with HF. The underlying mechanism may be related to the suppression of myocardial and renal neurohumoral activation.
Tolvaptan.[Pubmed:19644472]
Nat Rev Drug Discov. 2009 Aug;8(8):611-2.
In May 2009, Tolvaptan (Samsca; Otsuka), a selective vasopressin V(2) receptor antagonist, was approved by the US FDA for the treatment of clinically significant hypervolaemic and euvolaemic hyponatraemia.
7-Chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoyl-amino)benzoyl ]-2,3,4,5-tetrahydro-1H-1-benzazepine (OPC-41061): a potent, orally active nonpeptide arginine vasopressin V2 receptor antagonist.[Pubmed:10482466]
Bioorg Med Chem. 1999 Aug;7(8):1743-54.
We previously reported a series of benzazepine derivatives as orally active nonpeptide arginine vasopressin (AVP) V2 receptor antagonists. After the lead structure OPC-31260 was structurally evaluated and optimized, the introduction of the 7-Cl moiety on the benzazepine and 2-CH3 on the aminobenzoyl moiety enhanced its oral activity. The new AVP-V2 selective antagonist OPC-41061 was determined to be a potent and orally active agent.


