MozavaptanCAS# 137975-06-5 |
- Orbifloxacin
Catalog No.:BCC4689
CAS No.:113617-63-3
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Ciclopirox ethanolamine
Catalog No.:BCC4372
CAS No.:41621-49-2
- Carbenicillin
Catalog No.:BCC5192
CAS No.:4697-36-3
- Sulconazole Nitrate
Catalog No.:BCC4853
CAS No.:61318-91-0
- Sertaconazole nitrate
Catalog No.:BCC4716
CAS No.:99592-39-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 137975-06-5 | SDF | Download SDF |
| PubChem ID | 119369 | Appearance | Powder |
| Formula | C27H29N3O2 | M.Wt | 427.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | OPC-31260; OPC31260l | ||
| Solubility | DMSO : 6.2 mg/mL (14.50 mM; Need warming) H2O : < 0.1 mg/mL (insoluble) | ||
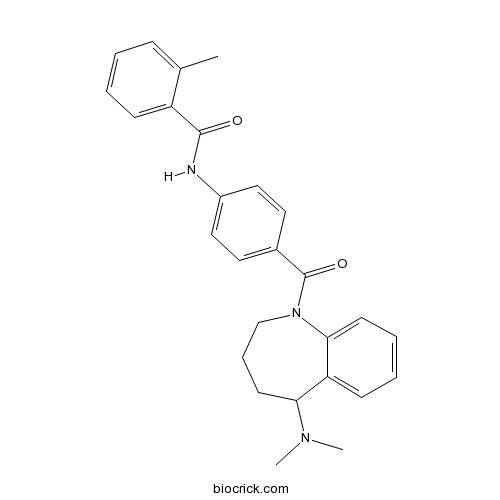
| Chemical Name | N-[4-[5-(dimethylamino)-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl]phenyl]-2-methylbenzamide | ||
| SMILES | CC1=CC=CC=C1C(=O)NC2=CC=C(C=C2)C(=O)N3CCCC(C4=CC=CC=C43)N(C)C | ||
| Standard InChIKey | WRNXUQJJCIZICJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mozavaptan (OPC31260) is a orally effective, nonpeptide vasopressin V2 receptor antagonist with an IC50 of 14 nM.In Vitro:Mozavaptan causes a competitive displacement of [3H]-arginine vasopressin (AVP) binding to both V1 and V2 receptors with IC50 values of 1.2 μM and 14 nM, respectively. The Kd of [3H]-AVP is reduced significantly in both rat liver and kidney in the presence of mozavaptan (Kd=1.1 nM in liver, Kd=1.38 nM in kidney)[1].In Vivo:Mozavaptan at doses of 10 to 100 μg/kg, i.v., inhibits the antidiuretic action of exogenously administered arginine vasopressin in water-loaded, alcohol-anaesthetized rats in a dose-dependent manner. Mozavaptan does not exert an antidiuretic activity suggesting that it is not a partial V2 receptor agonist. Mozavaptan dose-dependently increases urine flow and decreases urine osmolality after oral administration at doses of 1 to 30 mg/kg in normal conscious rats[1]. References: | |||||

Mozavaptan Dilution Calculator

Mozavaptan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.339 mL | 11.6948 mL | 23.3896 mL | 46.7792 mL | 58.4741 mL |
| 5 mM | 0.4678 mL | 2.339 mL | 4.6779 mL | 9.3558 mL | 11.6948 mL |
| 10 mM | 0.2339 mL | 1.1695 mL | 2.339 mL | 4.6779 mL | 5.8474 mL |
| 50 mM | 0.0468 mL | 0.2339 mL | 0.4678 mL | 0.9356 mL | 1.1695 mL |
| 100 mM | 0.0234 mL | 0.1169 mL | 0.2339 mL | 0.4678 mL | 0.5847 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mozavaptan is a novel competitive vasopressin receptor antagonist for both V1 and V2 receptors with IC50 of 1.2 μM and 14 nM, respectively.
- Dehydrotolvaptan
Catalog No.:BCC8932
CAS No.:137973-76-3
- CBB1007
Catalog No.:BCC4272
CAS No.:1379573-92-8
- CBB1003
Catalog No.:BCC5524
CAS No.:1379573-88-2
- Arillatose B
Catalog No.:BCN6196
CAS No.:137941-45-8
- ML 239
Catalog No.:BCC3987
CAS No.:1378872-36-6
- 6-O-Feruloylglucose
Catalog No.:BCN6195
CAS No.:137887-25-3
- Valsartan methyl ester
Catalog No.:BCC9189
CAS No.:137863-17-3
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- 2,3-Di(3',4'-methylenedioxybenzyl)-2-buten-4-olide
Catalog No.:BCN1576
CAS No.:137809-97-3
- Boeravinone E
Catalog No.:BCN4083
CAS No.:137787-00-9
- 12-O-Acetylrosmarinine
Catalog No.:BCN2125
CAS No.:137760-53-3
- 7-Methoxy-beta-carboline-1-propionic acid
Catalog No.:BCN2995
CAS No.:137756-13-9
- Atroscine
Catalog No.:BCN1941
CAS No.:138-12-5
- Mafenide
Catalog No.:BCC5237
CAS No.:138-39-6
- D-(-)-Salicin
Catalog No.:BCN6298
CAS No.:138-52-3
- Picrocrocine
Catalog No.:BCC8232
CAS No.:138-55-6
- Shikimic acid
Catalog No.:BCN6200
CAS No.:138-59-0
- Limonene
Catalog No.:BCN3797
CAS No.:138-86-3
- Decorticasine
Catalog No.:BCN2006
CAS No.:1380-03-6
- BET bromodomain inhibitor
Catalog No.:BCC6426
CAS No.:1380087-89-7
- KB SRC 4
Catalog No.:BCC6253
CAS No.:1380088-03-8
- EPZ5676
Catalog No.:BCC2215
CAS No.:1380288-87-8
- EPZ004777 HCl
Catalog No.:BCC4550
CAS No.:1380316-03-9
- Valeriandoid B
Catalog No.:BCN6754
CAS No.:1380399-57-4
Small cell gall bladder carcinoma complicated by syndrome of inappropriate secretion of antidiuretic hormone (SIADH) treated with mozavaptan.[Pubmed:23761568]
BMJ Case Rep. 2013 Jun 11;2013. pii: bcr-2013-010039.
Small cell gall bladder carcinoma (Scc-GB) is a very rare entity. Although some cases present with endocrine manifestations, paraneoplastic hyponatraemia has been reported in only one previous case. Recently, the antidiuretic hormone (ADH) receptor antagonist Mozavaptan has become available. Herein we report a case with Scc-GB complicated with syndrome of inappropriate secretion of antidiuretic hormone (SIADH) treated with Mozavaptan. A 47-year-old woman was referred to our hospital for hyponatraemia. Physical examination revealed elevated serum ADH, a gall bladder mass. She was clinically diagnosed with Scc-GB with SIADH as a paraneoplastic syndrome. Mozavaptan was used for SIADH. Serum sodium was quickly normalised after Mozavaptan treatment. Two months later, metastasis to the subcutis of the abdominal wall was observed. The metastatic nodule was resected, and small cell carcinoma (Scc) was identified pathologically. Mozavaptan was effective for improvement of hyponatraemia in this patient with Scc-GB complicated with SIADH.
Clinical implication of the antidiuretic hormone (ADH) receptor antagonist mozavaptan hydrochloride in patients with ectopic ADH syndrome.[Pubmed:21087977]
Jpn J Clin Oncol. 2011 Jan;41(1):148-52.
Ectopic antidiuretic hormone syndrome is a medical emergency characterized by dilutional hyponatremia. Clinical effectiveness of the vasopressin V2 receptor antagonist Mozavaptan was evaluated in 16 patients. In short-term (7-day) treatment with the drug, serum sodium concentration (mean +/- standard deviation) significantly (P = 0.002) increased from 122.8 +/- 6.7 to 133.3 +/- 8.3 mEq/l, and symptoms due to hyponatremia were improved. On the basis of these results, Mozavaptan (Physuline((R))) was approved as an orphan drug for the treatment of the syndrome in 2006 in Japan. During the 43 months following its launch, 100 patients have been treated with the drug; overall clinical effects of the drug were found similar to those of this clinical trial. Clinically, Mozavaptan may allow hyponatremic patients to be treated by aggressive cancer chemotherapy with platinum-containing drugs. Moreover, the drug may free patients from strict fluid-intake restrictions and thereby improve their quality of life.


