TetrodotoxinNa+ channel blocker CAS# 4368-28-9 |
Quality Control & MSDS
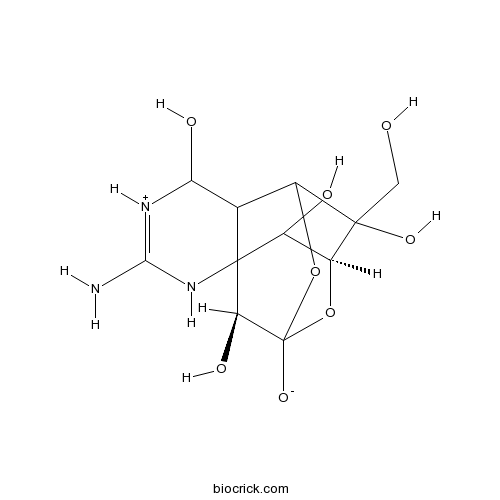
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4368-28-9 | SDF | Download SDF |
| PubChem ID | 443368 | Appearance | Powder |
| Formula | C11H17N3O8 | M.Wt | 319.27 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | TTx | ||
| Solubility | Soluble to 3 mM in acidic buffer (pH 4.8) | ||
| SMILES | C(C1(C2C3C([NH+]=C(NC34C(C1OC(C4O)(O2)[O-])O)N)O)O)O | ||
| Standard InChIKey | SLBCPBUVHCASIJ-UFHSVNPDSA-O | ||
| Standard InChI | InChI=1S/C11H16N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-19H,1H2,(H3,12,13,14)/q-1/p+1/t2?,3?,4?,5-,6?,7-,9?,10?,11?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tetrodotoxin, a poison from the puffer fish, at very low concentrations, blocks the action potential production through its selective inhibition of the sodium-carrying mechanism while keeping the potassium-carrying mechanism intact.Modulation of tetrodotoxin-resistant voltage-gated Na+ current (TTX-R INa) is a mechanism for sensitization of mammalian nociceptors. |
| Targets | Sodium Channel |
| In vitro | Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody.[Pubmed: 25913446]Biosens Bioelectron. 2015 Apr 18;71:256-260.
TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS.[Pubmed: 14155438]J Gen Physiol. 1964 May;47:965-74.Previous studies suggested that Tetrodotoxin, a poison from the puffer fish, blocks conduction of nerve and muscle through its rather selective inhibition of the sodium-carrying mechanism. |
| Kinase Assay | Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the type II pyrethroid deltamethrin.[Pubmed: 9495855]J Pharmacol Exp Ther. 1998 Mar;284(3):958-65.
|
| Structure Identification | Org Lett. 2015 Apr 27.Formal Synthesis of (±)-Tetrodotoxin via the Oxidative Amidation of a Phenol: On the Structure of the Sato Lactone.[Pubmed: 25915710]A formal total synthesis of (±)-Tetrodotoxin that relies on the bimolecular oxidative amidation of a phenol is described, and a structural correction of the Sato lactone, an important Tetrodotoxin intermediate, is provided. This work lays the foundation for an ultimate enantioselective synthesis. |

Tetrodotoxin Dilution Calculator

Tetrodotoxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1321 mL | 15.6607 mL | 31.3215 mL | 62.6429 mL | 78.3036 mL |
| 5 mM | 0.6264 mL | 3.1321 mL | 6.2643 mL | 12.5286 mL | 15.6607 mL |
| 10 mM | 0.3132 mL | 1.5661 mL | 3.1321 mL | 6.2643 mL | 7.8304 mL |
| 50 mM | 0.0626 mL | 0.3132 mL | 0.6264 mL | 1.2529 mL | 1.5661 mL |
| 100 mM | 0.0313 mL | 0.1566 mL | 0.3132 mL | 0.6264 mL | 0.783 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kobe0065
Catalog No.:BCC5290
CAS No.:436133-68-5
- JKC 363
Catalog No.:BCC6022
CAS No.:436083-30-6
- Ajmaline
Catalog No.:BCN3867
CAS No.:4360-12-7
- Fangchinoline
Catalog No.:BCN5956
CAS No.:436-77-1
- Diffractic Acid
Catalog No.:BCN8506
CAS No.:436-32-8
- (-)-Curine
Catalog No.:BCN2673
CAS No.:436-05-5
- 5-Hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one
Catalog No.:BCC8350
CAS No.:4354-76-1
- H-Arg(Tos)-OH
Catalog No.:BCC2867
CAS No.:4353-32-6
- L-5-Hydroxytryptophan
Catalog No.:BCC8106
CAS No.:4350-09-8
- K 41498
Catalog No.:BCC5867
CAS No.:434938-41-7
- Dacarbazine
Catalog No.:BCC1174
CAS No.:4342-03-4
- Nandrolone
Catalog No.:BCC9086
CAS No.:434-22-0
- MRS 2365
Catalog No.:BCC5879
CAS No.:436847-09-5
- Gentisin
Catalog No.:BCN7518
CAS No.:437-50-3
- Genkwanin
Catalog No.:BCN5488
CAS No.:437-64-9
- Xanthinol nicotinate
Catalog No.:BCC9191
CAS No.:437-74-1
- Crategolic acid
Catalog No.:BCN5487
CAS No.:4373-41-5
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- H-Thr(tBu)-OH
Catalog No.:BCC3106
CAS No.:4378-13-6
- 4-(4-Aminophenyl)morpholin-3-one
Catalog No.:BCC8650
CAS No.:438056-69-0
- SMI-4a
Catalog No.:BCC2233
CAS No.:438190-29-5
- Quercetin 3,3'-dimethyl ether
Catalog No.:BCN7781
CAS No.:4382-17-6
- Dihydrorobinetin
Catalog No.:BCN5489
CAS No.:4382-33-6
- Robtin
Catalog No.:BCN5490
CAS No.:4382-34-7
TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS.[Pubmed:14155438]
J Gen Physiol. 1964 May;47:965-74.
Previous studies suggested that Tetrodotoxin, a poison from the puffer fish, blocks conduction of nerve and muscle through its rather selective inhibition of the sodium-carrying mechanism. In order to verify this hypothesis, observations have been made of sodium and potassium currents in the lobster giant axons treated with Tetrodotoxin by means of the sucrose-gap voltage-clamp technique. Tetrodotoxin at concentrations of 1 x 10(-7) to 5 x 10(-9) gm/ml blocked the action potential but had no effect on the resting potential. Partial or complete recovery might have occurred on washing with normal medium. The increase in sodium conductance normally occurring upon depolarization was very effectively suppressed when the action potential was blocked after Tetrodotoxin, while the delayed increase in potassium conductance underwent no change. It is concluded that Tetrodotoxin, at very low concentrations, blocks the action potential production through its selective inhibition of the sodium-carrying mechanism while keeping the potassium-carrying mechanism intact.
Formal Synthesis of (+/-)-Tetrodotoxin via the Oxidative Amidation of a Phenol: On the Structure of the Sato Lactone.[Pubmed:25915710]
Org Lett. 2015 May 15;17(10):2424-7.
A formal total synthesis of (+/-)-Tetrodotoxin that relies on the bimolecular oxidative amidation of a phenol is described, and a structural correction of the Sato lactone, an important Tetrodotoxin intermediate, is provided. This work lays the foundation for an ultimate enantioselective synthesis.
Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the type II pyrethroid deltamethrin.[Pubmed:9495855]
J Pharmacol Exp Ther. 1998 Mar;284(3):958-65.
In both Tetrodotoxin-resistant (TTX-R) and Tetrodotoxin-sensitive (TTX-S) sodium channels, deltamethrin greatly prolonged the current during step depolarizing pulse and caused a large and prolonged slow tail current. The activation was shifted by 20 mV in the hyperpolarizing direction. These changes in channel kinetics account for the prolongation of action potential, membrane depolarization and spontaneous discharges in the deltamethrin-treated neurons. The slow tail current of TTX-S sodium channels rose and decayed slowly, showing a hook. By contrast, the slow tail current of TTX-R channels occurred quickly upon step repolarization. The slow tail current in deltamethrin-treated cells developed slowly during a depolarizing pulse, with a time constant in the order of several milliseconds. The percentages of sodium channels modified by deltamethrin were measured as a function of the deltamethrin concentration. The EC50 values were 0.53 microM and 0.37 microM for TTX-S and TTX-R sodium channels, respectively. However, when compared at the level of 5% modification, the potency of deltamethrin for TTX-R channels was 40 to 50 times higher than that for TTX-S channels. Deltamethrin-induced large and prolonged tail current was hardly reversed after prolonged washout with drug-free solution. However, after application of tetramethrin, it was converted into a much shorter tail current. Washout with solution devoid of tetramethrin and deltamethrin resulted in rapid reappearance of the deltamethrin-type tail current. These results suggest that the deltamethrin and tetramethrin share a binding site on the sodium channel and that the slow onset and offset of deltamethrin action are controlled by the rates at which deltamethrin moves and unbinds from the membrane lipid phase rather than by the rate of deltamethrin binding to the sodium channel site.
Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody.[Pubmed:25913446]
Biosens Bioelectron. 2015 Sep 15;71:256-260.
A monoclonal hybridoma cell named 5B9 against Tetrodotoxin (TTX) was obtained after fusion of myeloma SP2/0 cells with spleen cells isolated from the immunized Balb/c mice. The 5B9 monoclonal antibody (McAb) with high affinity (about 2.55 x 10(9)) is specific to TTX, and this McAb belongs to the immunoglobulin G (IgG) isotype. Finally, an enzyme-linked immunosorbent assay (ELISA) and colloidal gold immunoassay were established based on this McAb. The linear range of ELISA to detect TTX was 5-500 ng/mL, and the limit of detection (LOD) was 4.44 ng/mL. The average CV of intra- and inter-assay was less than 8%, with the samples recovery range of 70.93-99.99%. A competitive format colloidal gold strip was developed for detection of TTX in real samples, and the LOD for TTX is 20 ng/mL, and the assay time of the qualitative test can be finished in less than 10 min without any equipment. The result from test strip revealed that the test strip has a good agreement with those obtained from ELISA.
The protective action of tetrodotoxin and (+/-)-kavain on anaerobic glycolysis, ATP content and intracellular Na+ and Ca2+ of anoxic brain vesicles.[Pubmed:9076753]
Neuropharmacology. 1996;35(12):1743-52.
Because recent reports point to Na+ channel blockers as protective agents directed against anoxia-induced neuronal damage including protection of anaerobic glycolysis, the influences of Tetrodotoxin (TTX) and (+/-)-kavain on anoxic rat brain vesicles were investigated with respect to lactate synthesis, vesicular ATP content and cytosolic free Na+ and Ca2+ ([Na+]i, [Ca2+]i), both of the latter determined fluorometrically employing SBFI and FURA-2, respectively. After anoxia, basal lactate production was increased from 2.9 to 9.8 nmol lactate/min/mg protein. Although lactate synthesis seemed to be stable for at least 45 min of anoxia, as deduced from the linearity of lactate production, the ATP content declined continuously with a half life (tau 1/2) of 14.5 min, indicating that anaerobic glycolysis was insufficient to cover the energy demand of anoxic vesicles. Correspondingly, [Na+]i and [Ca2+]i increased persistently after anoxia by 22.1 mmol/l Na+ and 274.9 nmol/l Ca2+, determined 6.3 min after onset. An additional stimulation of vesicles with veratridine accelerated the drop of ATP (tau 1/2 = 5.1 min) and provoked a massive Na+ overload, which levelled off to 119 mmol/l Na+ within a few minutes. Concomitantly, [Ca2+]i increased linearly with a rate of 355 nmol Ca2+/l/min. Despite the massive perturbation of ion homeostasis, lactate production was unaffected during the first 8 min of veratridine stimulation. However, complete inhibition of lactate synthesis took place 30 min after veratridine was added. The Na+ channel blockers TTX and (+/-)-kavain, if applied before anoxia, preserved vesicular ATP content, diminished anoxia-induced increases in [Na+]i and [Ca2+]i and prevented both the veratridine-induced increases of [Na+]i and [Ca2+]i and the inhibition of lactate production. The data indicate a considerable Na+ influx via voltage-dependent Na+ channels during anoxia, which speeds up the decline in ATP and provokes an increase in [Ca2+]i. A massive Na+ and Ca2+ overload induced by veratridine failed to influence lactate synthesis directly, but initiated its inhibition.


