TetrahydrorhombifolineCAS# 3382-84-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3382-84-1 | SDF | Download SDF |
| PubChem ID | 15511175.0 | Appearance | Solid |
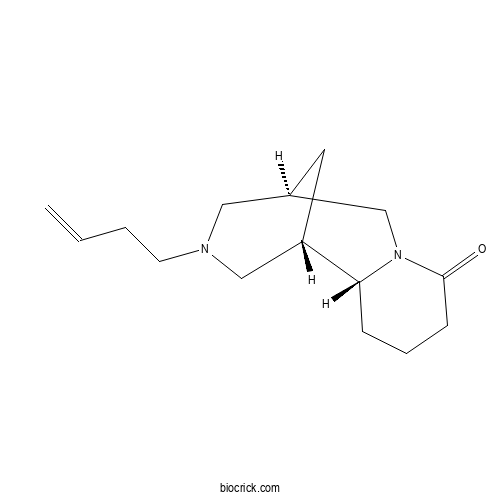
| Formula | C15H24N2O | M.Wt | 248.36 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2R,9R)-11-but-3-enyl-7,11-diazatricyclo[7.3.1.02,7]tridecan-6-one | ||
| SMILES | C=CCCN1CC2CC(C1)C3CCCC(=O)N3C2 | ||
| Standard InChIKey | OKTIETCHYDTVGN-HZSPNIEDSA-N | ||
| Standard InChI | InChI=1S/C15H24N2O/c1-2-3-7-16-9-12-8-13(11-16)14-5-4-6-15(18)17(14)10-12/h2,12-14H,1,3-11H2/t12-,13+,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetrahydrorhombifoline Dilution Calculator

Tetrahydrorhombifoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0264 mL | 20.1321 mL | 40.2641 mL | 80.5283 mL | 100.6603 mL |
| 5 mM | 0.8053 mL | 4.0264 mL | 8.0528 mL | 16.1057 mL | 20.1321 mL |
| 10 mM | 0.4026 mL | 2.0132 mL | 4.0264 mL | 8.0528 mL | 10.066 mL |
| 50 mM | 0.0805 mL | 0.4026 mL | 0.8053 mL | 1.6106 mL | 2.0132 mL |
| 100 mM | 0.0403 mL | 0.2013 mL | 0.4026 mL | 0.8053 mL | 1.0066 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- n-Butyl α-D-fructofuranoside
Catalog No.:BCX0392
CAS No.:80971-59-1
- Excoecafolin C
Catalog No.:BCX0391
CAS No.:1643370-00-6
- (2E,6E)-Farnesyl acetate
Catalog No.:BCX0390
CAS No.:4128-17-0
- 4,4'-(1,3-Dimethylbutylidene)diphenol
Catalog No.:BCX0389
CAS No.:6807-17-6
- 14,15,16-Trinorlabda-8(17),11-dien-13-oic acid
Catalog No.:BCX0388
CAS No.:917078-12-7
- Sclerone
Catalog No.:BCX0387
CAS No.:19638-58-5
- Malformin C
Catalog No.:BCX0386
CAS No.:59926-78-2
- Gnetuhainin I
Catalog No.:BCX0385
CAS No.:308105-06-8
- Asperazine
Catalog No.:BCX0384
CAS No.:198953-76-3
- Triptotriterpenic acid C
Catalog No.:BCX0383
CAS No.:123914-32-9
- Deacetylnomilin
Catalog No.:BCX0382
CAS No.:3264-90-2
- Oxytroflavoside G
Catalog No.:BCX0381
CAS No.:1391144-89-0
- 4''-O-Glucosyl-17α-deacetyltanghinin
Catalog No.:BCX0394
CAS No.:114612-75-8
- Levulinic acid
Catalog No.:BCX0395
CAS No.:123-76-2
- 17α-Deacetyltanghinin
Catalog No.:BCX0396
CAS No.:111614-46-1
- 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate
Catalog No.:BCX0397
CAS No.:148757-94-2
- Disporopsin
Catalog No.:BCX0398
CAS No.:1430334-05-6
- 8-Methyldisporopsin
Catalog No.:BCX0399
CAS No.:1671098-51-3
- 8-Methyldisporopsin 4'-methyl ether
Catalog No.:BCX0400
CAS No.:1589545-88-9
- 8-Methyl-2'-deoxydisporopsin
Catalog No.:BCX0401
CAS No.:1671098-52-4
- Disporopsin 4'-methyl ether
Catalog No.:BCX0402
CAS No.:1671098-53-5
- Ichanexic acid
Catalog No.:BCX0403
CAS No.:1044818-57-6
- 3''-Methoxycentrolobol
Catalog No.:BCX0404
CAS No.:811471-19-9
- Kaempferol 7-O-(4''-O-methyl)glucoside
Catalog No.:BCX0405
CAS No.:1092795-48-6
Alkaloid profile of leaves and seeds of Lupinus hintonii C. P. Smith.[Pubmed:12064721]
Z Naturforsch C J Biosci. 2002 Mar-Apr;57(3-4):243-7.
L. hintonii C. P. Smith grows in the Central Highland forests of Mexico at altitudes between 2800 m to 3200 m above see level. Members of the genus Lupinus produce quinolizidine alkaloids as main chemical defensive compounds against herbivores. Surprisingly alkaloid profiles are rather constant within this species, while substantial variation was found when compared to morphologically closely related other taxa. As part of a phytochemical project on Mexican wild lupins, we report on the alkaloid profiles of seeds and leaves of L. hintonii. 19 alkaloids could be identified by capillary GLC-MS. Six major alkaloids occurred in leaves and seeds: 13-hydroxylupanine (28% and 45% respectively), Tetrahydrorhombifoline (31% and 23% respectively), angustifoline (2% and 4% respectively), lupanine (7% and 5% respectively), 13alpha-tigloyloxylupanine (19% and 5% respectively) and 4alpha-angeloyl-3beta-hydroxylupanine (9% and 2%). This chemical pattern resembles that of the North American lupin L. floribundus.
Biochemical and partial molecular characterization of bitter and sweet forms of Lupinus angustifolius, an experimental model for study of molecular regulation of quinolizidine alkaloid biosynthesis.[Pubmed:11045450]
Chem Pharm Bull (Tokyo). 2000 Oct;48(10):1458-61.
The bitter and sweet forms of a plant species differing with alkaloid contents may provide a model system for investigation of alkaloid biosynthesis at a molecular level. The pattern and concentration of quinolizidine alkaloids were determined by capillary GC-MS in bitter and sweet plants of Lupinus angustifolius. Bitter plant contained lupanine, 13alpha-hydroxylupanine, angustifoline, alpha-isolupanine, Tetrahydrorhombifoline, and ester-derivatives of 13alpha-hydroxylupanine. In contrast, no alkaloid was detected in sweet plant. The enzymatic activity of acyltransferase for formation of 13alpha-tigloyloxylupanine was similar or even higher in the cell-free extracts of sweet plant than that in bitter plant. These results suggest that the biosynthetic step(s) of ring closure forming the initial cyclic alkaloid, lupanine, from cadaverine is presumably blocked in sweet plant, and that the later steps for modification of the cyclized alkaloids are not altered. We hypothesized that the gene(s) encoding enzyme(s) for ring-closure step might be repressed in sweet plant, and that the expression might take place only in bitter plant. To isolate the genes specifically expressed in bitter plant, cDNA-amplified fragment length polymorphism (cDNA-AFLP) analysis was carried out. However, no bitter-specific gene was isolated, suggesting that alkaloid biosynthesis in sweet plant may be down-regulated at a post-transcriptional level.
Accumulation of quinolizidine alkaloids in plants and cell suspension cultures: genera lupinus, cytisus, baptisia, genista, laburnum, and sophora.[Pubmed:17404991]
Planta Med. 1983 Aug;48(8):253-7.
The patterns of quinolizidine alkaloids in cell cultures of 10 species of Fabaceae were analyzed by high-resolution GLC and GLC-MS and compared with the alkaloids present in the leaves of the respective plants. Lupanine was produced in all 10 cell suspension cultures as the main alkaloid. It was accompanied by sparteine, Tetrahydrorhombifoline, 17-oxosparteine, 13-hydroxylupanine, 4-hydroxylupanine, 17-oxolupanine, and 13-hydroxylupanine esters as minor alkaloids in some species. The alkaloid patterns of the plants differed markedly in that alpha-pyridone alkaloids were the major alkaloids in the genera Cytisus, Genista, Laburnum and Sophora but were not accumulated in the cell cultures. These data further support the assumption that the pathway leading to lupanine is the basic pathway of quinolizidine alkaloids biosynthesis and that the other alkaloids are derived from lupanine.


