DeacetylnomilinCAS# 3264-90-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3264-90-2 | SDF | Download SDF |
| PubChem ID | 146115918 | Appearance | Powder |
| Formula | C26H32O8 | M.Wt | 472.5 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
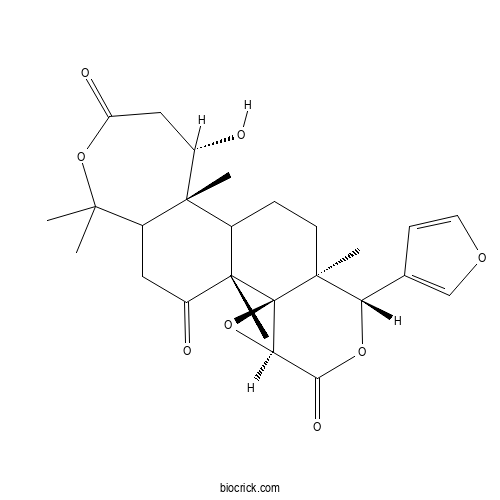
| Chemical Name | (1R,2R,4S,7S,8S,12R,13S)-7-(furan-3-yl)-13-hydroxy-1,8,12,17,17-pentamethyl-3,6,16-trioxapentacyclo[9.9.0.02,4.02,8.012,18]icosane-5,15,20-trione | ||
| SMILES | CC1(C2CC(=O)C3(C(C2(C(CC(=O)O1)O)C)CCC4(C35C(O5)C(=O)OC4C6=COC=C6)C)C)C | ||
| Standard InChIKey | HWAJASVMTDEFJN-RUQWRMCFSA-N | ||
| Standard InChI | InChI=1S/C26H32O8/c1-22(2)15-10-17(28)25(5)14(24(15,4)16(27)11-18(29)33-22)6-8-23(3)19(13-7-9-31-12-13)32-21(30)20-26(23,25)34-20/h7,9,12,14-16,19-20,27H,6,8,10-11H2,1-5H3/t14?,15?,16-,19-,20+,23-,24+,25-,26+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Deacetylnomilin Dilution Calculator

Deacetylnomilin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1164 mL | 10.582 mL | 21.164 mL | 42.328 mL | 52.9101 mL |
| 5 mM | 0.4233 mL | 2.1164 mL | 4.2328 mL | 8.4656 mL | 10.582 mL |
| 10 mM | 0.2116 mL | 1.0582 mL | 2.1164 mL | 4.2328 mL | 5.291 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4233 mL | 0.8466 mL | 1.0582 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4233 mL | 0.5291 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxytroflavoside G
Catalog No.:BCX0381
CAS No.:1391144-89-0
- Diferuloylputrescine
Catalog No.:BCX0380
CAS No.:42369-86-8
- Versicolactone B
Catalog No.:BCX0379
CAS No.:108885-62-7
- Madolin U
Catalog No.:BCX0378
CAS No.:327185-00-2
- Calquiquelignan E
Catalog No.:BCX0377
CAS No.:1292294-31-5
- Calquiquelignan D
Catalog No.:BCX0376
CAS No.:1928715-38-1
- Didemethoxycyclocurcumin
Catalog No.:BCX0375
CAS No.:1042441-12-2
- 3β,5β,6α-Trihydroxy-7-megastigmen-9-one 3-O-glucoside
Catalog No.:BCX0374
CAS No.:1380443-06-0
- Salcolin B
Catalog No.:BCX0373
CAS No.:369390-52-3
- Triptriolide
Catalog No.:BCX0372
CAS No.:137131-18-1
- 8-Demethoxyschinilenol
Catalog No.:BCX0371
CAS No.:144398-46-9
- 9,10-Dihydroxymegastigma-4,7-dien-3-one
Catalog No.:BCX0370
CAS No.:349642-88-2
- Triptotriterpenic acid C
Catalog No.:BCX0383
CAS No.:123914-32-9
- Asperazine
Catalog No.:BCX0384
CAS No.:198953-76-3
- Gnetuhainin I
Catalog No.:BCX0385
CAS No.:308105-06-8
- Malformin C
Catalog No.:BCX0386
CAS No.:59926-78-2
- Sclerone
Catalog No.:BCX0387
CAS No.:19638-58-5
- 14,15,16-Trinorlabda-8(17),11-dien-13-oic acid
Catalog No.:BCX0388
CAS No.:917078-12-7
- 4,4'-(1,3-Dimethylbutylidene)diphenol
Catalog No.:BCX0389
CAS No.:6807-17-6
- (2E,6E)-Farnesyl acetate
Catalog No.:BCX0390
CAS No.:4128-17-0
- Excoecafolin C
Catalog No.:BCX0391
CAS No.:1643370-00-6
- n-Butyl α-D-fructofuranoside
Catalog No.:BCX0392
CAS No.:80971-59-1
- Tetrahydrorhombifoline
Catalog No.:BCX0393
CAS No.:3382-84-1
- 4''-O-Glucosyl-17α-deacetyltanghinin
Catalog No.:BCX0394
CAS No.:114612-75-8
Chemical profile of persian lime seeds (Citrus Limettioides T.): Focus on limonoids and polyphenols.[Pubmed:38088731]
An Acad Bras Cienc. 2023 Dec 11;95(suppl 2):e20230322.
Citrus fruit industrial processing generates tons of waste composed of peels, seeds and pulp. Incorrect disposal of these residues may harm the environment. The extraction of oil and bioactive compounds from citrus fruit seeds may be considered a sustainable alternative to the disposal of waste by the citrus agroindustry. In order to provide safe disposal of citrus waste an evaluation of its composition is necessary. Here we report the results of the application of a methodology to evaluate the composition the seeds of Citrus limettioides. In the first step, extraction with supercritical carbon dioxide was used. This work allowed the isolation and identification of four aglycone-type limonoids by High Performance Liquid Chromatography and Nuclear Magnetic Resonance, identified as limonin, nomilin, Deacetylnomilin, and obacunone. In addition, six other polar limonoids and two glycosyl flavonoids were identified by HPLC-ESI/MS/MS.
Evaluation of Pomelo Seed Extracts as Natural Antioxidant, Antibacterial, Herbicidal Agents, and Their Functional Components.[Pubmed:34651409]
Chem Biodivers. 2021 Dec;18(12):e2100679.
Pomelo seeds (PS) are important by-product of pomelo fruits (Citrus grandis Osbeck). The value-added utilization of PS remains highly challenged. This study aimed to investigate the utilization potential of PS as natural antioxidant, antibacterial, herbicidal agents, and their functional components. The ethanolic extract (EE) of PS and its four fractions as PEE (petroleum ether extract), AcOEtE (ethyl acetate extract), BTE (butanol extract), and WE (water extract), were prepared and biologically evaluated. BTE exhibited the best antioxidant activity among all these extracts, in both ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) and FRAP (ferric reducing antioxidant power) assays. AcOEtE was superior to other extracts in herbicidal assay against both Festuca elata Keng (IC(50) of 0.48 mg mL(-1) ) and Amaranthus retroflexus L. (IC(50) of 0.94 mg mL(-1) ). Meanwhile, both AcOEtE and BTE demonstrated inhibitory effects against Bacillus subtilis, Escherichia coli, and Xanthomonas citri subsp. citri, with MIC ranging 2.5-5.0 mg mL(-1) . Furthermore, the primary chemical components involving naringin, Deacetylnomilin, limonin, nomilin, and obacunone, were quantified in all these extracts. PCA (principal component analysis) suggested that naringin might highly contribute to the antioxidant activity of PS, and the herbicidal activity should be ascribed to limonoids. This study successfully identified AcOEtE and BTE as naturally occurring antioxidant, antibacterial, and herbicidal agents, showing application potential in food and cosmetics industries, and organic farming agriculture.
Research on Biomarkers of Different Growth Periods and Different Drying Processes of Citrus wilsonii Tanaka Based on Plant Metabolomics.[Pubmed:34335665]
Front Plant Sci. 2021 Jul 14;12:700367.
Fruit of Citrus wilsonii Tanaka called as "Xiang yuan" in Chinese, which means fragrant and round. It was widely used in the pharmaceutical and food industries. This fruit has well-known health benefits such as antioxidant, radical scavenging, and anti-inflammatory. Naringin, Deacetylnomilin, citric acid, limonin, and nomilin were the characteristic components of Citrus wilsonii Tanaka. Although the fruit of Citrus wilsonii Tanaka possessed many applications, there was a lack of research on the growth period and drying process. In this study, plant metabolomics was used to analyze the biomarkers of the growth period, and appearance indicators and metabolites abundance were combined for the analysis of change regularities of the growth period. The representative differential metabolites of naringin, citric acid, and limonin were screened out, and the abundance of these components was relatively highest in the middle of the growth period. Therefore, the fruit of Citrus wilsonii Tanaka should be harvested before it turned yellow completely, which could effectively ensure the content of potential active ingredients. In the comparison of different drying methods, citric acid and naringin were considered to be representative differential components, but limonoids were relatively stable and not easily affected by drying methods. Naringin was an index component that could not only be reflected the maturity but also related to different drying methods. Considering its physical and chemical properties and its position, naringin had the potential to be a biomarker of Citrus wilsonii Tanaka.
Interaction of selected terpenoids with two SARS-CoV-2 key therapeutic targets: An in silico study through molecular docking and dynamics simulations.[Pubmed:34116362]
Comput Biol Med. 2021 Jul;134:104538.
The outbreak of COVID-19 disease caused by SARS-CoV-2, along with the lack of targeted medicaments, forced the scientific world to search for new antiviral formulations. In the current emergent situation, drug repurposing of well-known traditional and/or approved drugs could be the most effective strategy. Herein, through computational approaches, we aimed to screen 14 natural compounds from limonoids and terpenoids class for their ability to inhibit the key therapeutic target proteins of SARS-CoV-2. Among these, some limonoids, namely Deacetylnomilin, ichangin and nomilin, and the terpenoid beta-amyrin provided good interaction energies with SARS-CoV-2 3CL hydrolase (Mpro) in molecular dynamic simulation. Interestingly, Deacetylnomilin and ichangin showed direct interaction with the catalytic dyad of the enzyme so supporting their potential role in preventing SARS-CoV-2 replication and growth. On the contrary, despite the good affinity with the spike protein RBD site, all the selected phytochemicals lose contact with the amino acid residues over the course of 120ns-long molecular dynamics simulations therefore suggesting they scarcely can interfere in SARS-CoV-2 binding to the ACE2 receptor. The in silico analyses of docking score and binding energies, along with predicted pharmacokinetic profiles, indicate that these triterpenoids might have potential as inhibitors of SARS-CoV-2 Mpro, recommending further in vitro and in vivo investigations for a complete understanding and confirmation of their inhibitory potential.
Secondary metabolites of ponderosa lemon (Citrus pyriformis) and their antioxidant, anti-inflammatory, and cytotoxic activities.[Pubmed:21950163]
Z Naturforsch C J Biosci. 2011 Jul-Aug;66(7-8):385-93.
Column chromatography of the dichloromethane fraction from an aqueous methanolic extract of fruit peel of Citrus pyriformis Hassk. (Rutaceae) resulted in the isolation of seven compounds including one coumarin (citropten), two limonoids (limonin and Deacetylnomilin), and four sterols (stigmasterol, ergosterol, sitosteryl-3-beta-D-glucoside, and sitosteryl-6'-O-acyl-3-beta-D-glucoside). From the ethyl acetate fraction naringin, hesperidin, and neohesperidin were isolated. The dichloromethane extract of the defatted seeds contained three additional compounds, nomilin, ichangin, and cholesterol. The isolated compounds were identified by MS (EI, CI, and ESI), 1H, 13C, and 2D-NMR spectral data. The limonoids were determined qualitatively by LC-ESI/MS resulting in the identification of 11 limonoid aglycones. The total methanolic extract of the peel and the petroleum ether, dichloromethane, and ethyl acetate fractions were screened for their antioxidant and anti-inflammatory activities. The ethyl acetate fraction exhibited a significant scavenging activity for DPPH free radicals (IC50 = 132.3 microg/mL). The petroleum ether fraction inhibited 5-lipoxygenase with IC50 = 30.6 microg/mL indicating potential anti-inflammatory properties. Limonin has a potent cytotoxic effect against COS7 cells [IC50 = (35.0 +/- 6.1) microM] compared with acteoside as a positive control [IC50 = (144.5 +/- 10.96) microM].
Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines.[Pubmed:19782062]
Eur J Pharmacol. 2010 Jan 25;626(2-3):139-45.
P-glycoprotein (P-gp), a membrane transporter encoded by the MDR1 gene in human cells, mediates drug efflux from cells and plays a major role in causing multidrug resistance; which is one of the most accepted mechanisms for failure of chemotherapy in cancer treatment. In this study, we investigated the effects of nine naturally occurring compounds isolated from Citrus jambhiri Lush and Citrus pyriformis Hassk (Rutaceae) for their potential to modulate the activity of P-gp in the multidrug-resistant human leukaemia cell line CEM/ADR5000. Limonin, Deacetylnomilin, hesperidin, neohesperidin, stigmasterol and ss-sitosterol-O-glucoside inhibited the efflux of the P-gp substrate rhodamine 123 in a concentration-dependent manner. Some of these compounds were more active than verapamil, which was used as a positive control. Treatment of drug-resistant Caco-2 cells with the most active C. jambhiri and C. pyriformis compounds increased their sensitivity to doxorubicin and completely reversed doxorubicin resistance, which agrees with a decreased P-gp activity. Limonin was the most potent P-glycoprotein inhibitor - when it was applied at a non-toxic concentration of 20 microM, it significantly enhanced doxorubicin cytotoxicity 2.98-fold (P<0.001) and 2.2-fold (P<0.001) in Caco2 and CEM/ADR5000 cells, respectively. These isolated Citrus compounds could be considered as good candidates for the development of novel P-gp/MDR1 reversal agents which may enhance the accumulation and efficacy of chemotherapy agents.
Red Mexican grapefruit: a novel source for bioactive limonoids and their antioxidant activity.[Pubmed:17542482]
Z Naturforsch C J Biosci. 2007 Mar-Apr;62(3-4):179-88.
Citrus limonoids have shown to inhibit the growth of cancer in colon, lung, mouth, stomach and breast in animal and cell culture studies. For the first time in the present study, an attempt has been made to isolate antioxidant fractions and five limonoids from red Mexican grapefruit seeds. Defatted seed powder was successively extracted with hexane, ethyl acetate (EtOAc), acetone, methanol (MeOH) and MeOH/water and the extracts were concentrated under vacuum. Radical scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) and total phenolic content were also measured for comparison with the antioxidant capacity in the phosphomolybdenum method for the above extracts. Acetone and MeOH extracts, respectively, showed the highest (85.7%) and lowest (53.3%) radical scavenging activity, at 500 ppm. The total phenolic contents were found to be highest in the acetone extract (15.94%) followed by the MeOH extract (5.92%), ethyl acetate extract (5.54%) and water extract (5.26%). Antioxidant capacity of the extracts as equivalents to ascorbic acid (micromol/g of the extract) was in the order, EtOAc extract > acetone extract > water extract > methanol extract. Furthermore, the EtOAC and acetone extracts were loaded onto silica gel columns to obtain four limonoid aglycons. MeOH fraction was loaded onto a dowex-50 and sepabeads resin column to obtain a limonoid glucoside. The purity of the isolated five compounds was analyzed by HPLC using a C18 column and UV detection at 210 nm. Finally, the structures of the compounds were identified as obacunone, nomilin, limonin, Deacetylnomilin (DAN) and limonin-17-beta-D-glucopyranoside (LG) using 1H and 13C NMR studies.
Antiproliferative effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells.[Pubmed:17176224]
Nutr Cancer. 2006;56(1):103-12.
Limonoids, a family of triterpenoids with putative anticancer properties, occur in fruits as glucosides and aglycones. Both highly purified forms were isolated from seeds and molasses of citrus fruits and tested for toxic effects against two human cancer cell lines, SH-SY5Y neuroblastoma and Caco-2 colonic adenocarcinoma, and a noncancerous mammalian epithelial Chinese hamster ovary (CHO) cells. Viability, as quantified by 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium reduction and light microscopy, was shortened significantly (P < 0.001) in cancer cells exposed to aglycones, viz., limonin, nomilin, obacunone, and Deacetylnomilin. SH-SY5Y cells were more sensitive than Caco-2 cells to the limonoids, whereas noncancerous CHO cells showed hardly any change in cell numbers or cell morphology. Aglycone toxicity was dose dependent, but below the killing potential of glucosides. This observation correlated with a slower rate of induction of caspase 3/7 activity by aglycones. A flow cytometric analysis of SH-SY5Y cells treated with glucosides and aglycones showed an increased ploidy, which is consistent with enhancing chromosomal abnormalities. The results confirm that limonoids exert a strong multifaceted lethal action against cancer cells, but are relatively ineffective against CHO cells. Of the two, metabolites derived from glucosides are the more likely progenitors of an apoptosis response in situ.
Mass spectrometry and tandem mass spectrometry of citrus limonoids.[Pubmed:14710824]
Anal Chem. 2003 Oct 15;75(20):5451-60.
Methods for atmospheric pressure chemical ionization tandem mass spectrometry (APCI-MS/MS) of citrus limonoid aglycones and electrospray ionization tandem mass spectrometry (ESI-MS/MS) of limonoid glucosides are reported. The fragmentation patterns of four citrus limonoid aglycones (limonin, nomilin, obacunone, and Deacetylnomilin) and six limonoid glucosides, that is, limonin 17-beta-D-glucopyranoside (LG), nomilin 17-beta-D-glucopyranoside (NG), nomilinic acid 17-beta-D-glucopyranoside (NAG), deacetyl nomilinic acid 17-beta-D-glucopyranoside (DNAG), obacunone 17-beta-D-glucopyranoside (OG), and obacunoic acid 17-beta-D-glucopyranoside (OAG) were investigated using a quadruple mass spectrometer in low-energy collisionally activated dissociation (CAD). The four limonoid aglycones and four limonoid glucosides (LG, OG, NAG, and DNAG) were purified from citrus seeds; the other two limonoid glucosides (NG and OAG) were tentatively identified in the crude extract of grapefruit seeds by ESI mass spectrometry in both positive and negative ion analysis. Ammonium hydroxide or acetic acid was added to the mobile phase to facilitate ionization. During positive ion APCI analysis of limonoid aglycones, protonated molecular ion, [M + H]+, or adduct ion, [M + NH3 + H]-, was formed as base peaks when ammonium hydroxide was added to the mobile phase. Molecular anions or adduct ions with acetic acid ([M + HOAc - H] and [M + HOAc]-) or a deprotonated molecular ion were produced during negative ion APCI analysis of limonoid aglycones, depending on the mobile-phase modifier used. Positive ion ESI-MS of limonoid glucosides produced adduct ions of [M + H + NH3]+, [M + Na]+, and [M + K]+ when ammonium hydroxide was added to the mobile phase. After collisionally activated dissociation (CAD) of the limonoid aglycone molecular ions in negative ion APCI analysis, fragment ions indicated structural information of the precursor ions, showing the presence of methyl, carboxyl, and oxygenated ring structure. CAD of the adduct ion [M + H + NH3]+ of limonoid glucosides produced the aglycone moiety corresponding to each glucoside. The combination of mass spectrometry and tandem mass spectrometry provides a powerful technique for identification and characterization of citrus limonoids.
Electron ionization mass spectrometry of citrus limonoids.[Pubmed:14608622]
Rapid Commun Mass Spectrom. 2003;17(22):2517-22.
Electron ionization (EI) mass spectrometry was used to differentiate four structurally closely related citrus limonoid aglycones, including limonin, nomilin, obacunone, and Deacetylnomilin. The limonoids were isolated and purified from citrus seeds. Structures of major fragment ions were elucidated by high-resolution mass spectrometry (HRMS) and fragmentation pathways were proposed. The fragmentation patterns observed in the EI spectra can be used as important references for the positive characterization of limonoid aglycones.
Limonoids from Citrus reticulata.[Pubmed:12710721]
Z Naturforsch C J Biosci. 2003 Mar-Apr;58(3-4):165-70.
The seeds of Citrus reticulata afforded the new limonoid derivative, isolimonexic acid methyl ether, in addition to the previously isolated limonin, Deacetylnomilin, obacunone and ichangin. The structure elucidation was achieved primarily through 1D and 2-D-NMR analyses. The marginal antimalarial activity of isolimonexic acid methyl ether is reported.


