TetracaineCAS# 94-24-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94-24-6 | SDF | Download SDF |
| PubChem ID | 5411 | Appearance | Powder |
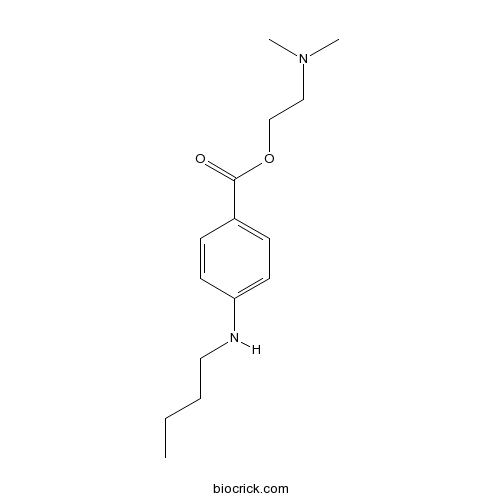
| Formula | C15H24N2O2 | M.Wt | 264.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(dimethylamino)ethyl 4-(butylamino)benzoate | ||
| SMILES | CCCCNC1=CC=C(C=C1)C(=O)OCCN(C)C | ||
| Standard InChIKey | GKCBAIGFKIBETG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetracaine Dilution Calculator

Tetracaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7821 mL | 18.9107 mL | 37.8215 mL | 75.643 mL | 94.5537 mL |
| 5 mM | 0.7564 mL | 3.7821 mL | 7.5643 mL | 15.1286 mL | 18.9107 mL |
| 10 mM | 0.3782 mL | 1.8911 mL | 3.7821 mL | 7.5643 mL | 9.4554 mL |
| 50 mM | 0.0756 mL | 0.3782 mL | 0.7564 mL | 1.5129 mL | 1.8911 mL |
| 100 mM | 0.0378 mL | 0.1891 mL | 0.3782 mL | 0.7564 mL | 0.9455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chlorpropamide
Catalog No.:BCC4647
CAS No.:94-20-2
- Benzyl 4-hydroxybenzoate
Catalog No.:BCC8869
CAS No.:94-18-8
- Sodium 4-amiropparaty Hyalrate
Catalog No.:BCC3855
CAS No.:94-16-6
- Propylparaben
Catalog No.:BCN8416
CAS No.:94-13-3
- Benzocaine
Catalog No.:BCC4636
CAS No.:94-09-7
- Synephrine
Catalog No.:BCN6308
CAS No.:94-07-5
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- BI 6015
Catalog No.:BCC6249
CAS No.:93987-29-2
- ACTB-1003
Catalog No.:BCC5587
CAS No.:939805-30-8
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
- Butylparaben
Catalog No.:BCN8418
CAS No.:94-26-8
- Benzyl nicotinate
Catalog No.:BCC8874
CAS No.:94-44-0
- 2-Amino-6-ethoxybenzothiazole
Catalog No.:BCC8541
CAS No.:94-45-1
- Piperine
Catalog No.:BCN1018
CAS No.:94-62-2
- N,N'-Bis(salicylidene)ethylenediamine
Catalog No.:BCC9064
CAS No.:94-93-9
- NVP-BHG712
Catalog No.:BCC3963
CAS No.:940310-85-0
- Suplatast Tosylate
Catalog No.:BCC4961
CAS No.:94055-76-2
- Poliumoside
Catalog No.:BCN1204
CAS No.:94079-81-9
- R-7128
Catalog No.:BCC1880
CAS No.:940908-79-2
- SB743921
Catalog No.:BCC4559
CAS No.:940929-33-9
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
- 1'-Acetonaphthone
Catalog No.:BCC8446
CAS No.:941-98-0
Efficacy and Safety of COX-2 Inhibitor Parecoxib for Rigid Cystoscopy-related Pain Management in Male Patients: A Prospective, Randomized and Controlled Study.[Pubmed:30868497]
Curr Med Sci. 2019 Feb;39(1):94-98.
Using anesthetic gel may not sufficiently exclude pain perception during and after cystoscopy in male patients. To evaluate the analgesic efficacy and safety of intramuscular parecoxib (40 mg) for outpatient-based rigid cystoscopy, we performed a prospective, randomized and controlled study. Consecutive male patients requiring diagnostic cystoscopy in our hospital were divided into group A (1% Tetracaine gel, n=50) and group B (parecoxib, n=51) at random. Patients received intramuscular injections of either 2 mL sterile saline in group A or 40 mg parecoxib in group B 30 min before the procedure. Tetracaine gel was injected into the urethra 3 min before the procedure in group A, with patients receiving plain lubricant gel in group B at the same time. Cystoscopy-associated pain levels were evaluated using the Visual Analog Score (VAS) during the procedure. Post-procedure urethral pain and complications were recorded and analyzed. The results showed that male patients experienced significantly less pain in group B than in group A (2.70+/-1.36 vs. 3.56+/-1.74, P=0.008). The percentage of patients with dysuria pain was not significantly different between the two groups. In addition, 24 h after cystoscopy, the patients with no previous experience of cystoscopy were more likely to declare urethral pain (59.2% vs. 33.3%, P=0.012, relative risk=1.78). No difference was observed in analgesic-related complications between the two groups. We conclude that intramuscular injection of 40 mg parecoxib may improve comfort for male patients undergoing rigid cystoscopy.
Reduction of oculocardiac reflex with Tetracaine eye drop in strabismus surgery.[Pubmed:30831045]
Strabismus. 2019 Mar;27(1):1-5.
INTRODUCTION: Recently, to reduce the incidence of oculocardiac reflex (OCR) in strabismus surgery, retrobulbar block and anticholinergic drugs or local anesthesia are also used. The present study evaluated the effects of Tetracaine eye drop as a topical nerve blocker on OCR during strabismus surgery. METHODS AND MATERIALS: In this randomized trial, 70 strabismus surgery candidates were randomly divided into placebo or synthetic teardrop (E) and Tetracaine eye drop (T) groups, so 3 drops of each solution were dropped in four directions of patients' eye immediately after applying anesthesia and before surgery. The incidence and severity of OCR during the stages of muscle release and incision (cutting), hemodynamic changes, the required time for OCR recovery and atropine dose were assessed. RESULTS: OCR was more seen in release phase compared to cutting phase. There were no significant differences between two group regarding the incidence and severity of OCR in the release phase (p > 0.05), but the incidence and severity of OCR in the cutting phase was more in group E than group T (p = 0.02, for both). The duration of OCR improvement (p-value = 0.74) and Atropine consumption (p-value = 0.92) did not differ between the groups. CONCLUSION: Tetracaine eye drop only reduces the incidence and severity of OCR during the incision stage of strabismus surgery.
Anesthetic Efficacy of Intranasal 3% Tetracaine plus 0.05% Oxymetazoline (Kovanaze) in Maxillary Teeth.[Pubmed:30803532]
J Endod. 2019 Mar;45(3):257-262.
INTRODUCTION: Needle-free anesthetic delivery is a promising alternative to traditional anesthetic routes of administration. The purpose of this study was to determine the patient preference for and pulpal anesthetic efficacy of a 3% Tetracaine plus 0.05% oxymetazoline (Kovanaze) nasal spray in maxillary lateral incisors and first premolars. METHODS: Fifty adult subjects randomly received a 3% Tetracaine plus 0.05% oxymetazoline (Kovanaze) nasal spray and mock infiltration or a mock nasal spray and 2% lidocaine with 1:100,000 epinephrine infiltration at the maxillary lateral incisor or first premolar in 2 appointments spaced at least 1 week apart in a single-blind cross-over design. Pulpal anesthesia was evaluated with an electric pulp tester. Side effects and subject preferences were also recorded. RESULTS: Anesthetic success was significantly lower for the Kovanaze nasal spray and mock infiltration (22%-37%) than for the mock nasal spray and lidocaine infiltration (89%-91%). Subjects reported more unwanted effects (nasal drainage and congestion, burning, pressure, and sinus congestion) after the Kovanaze nasal spray and mock infiltration than the mock spray and maxillary infiltration. Before participating in the study, more subjects (56%) preferred the nasal spray route versus a standard infiltration (44%). After experiencing both routes of administration, 100% of subjects preferred the standard infiltration. CONCLUSIONS: The 3% Tetracaine plus 0.05% oxymetazoline (Kovanaze) nasal spray provided significantly less successful pulpal anesthesia than the lidocaine infiltration, was less preferable, and caused more unwanted effects.


